Changes from the WA Museum Checklist from April 2018 (Updated September 2018)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lake Pinaroo Ramsar Site
Ecological character description: Lake Pinaroo Ramsar site Ecological character description: Lake Pinaroo Ramsar site Disclaimer The Department of Environment and Climate Change NSW (DECC) has compiled the Ecological character description: Lake Pinaroo Ramsar site in good faith, exercising all due care and attention. DECC does not accept responsibility for any inaccurate or incomplete information supplied by third parties. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. Readers should seek appropriate advice about the suitability of the information to their needs. © State of New South Wales and Department of Environment and Climate Change DECC is pleased to allow the reproduction of material from this publication on the condition that the source, publisher and authorship are appropriately acknowledged. Published by: Department of Environment and Climate Change NSW 59–61 Goulburn Street, Sydney PO Box A290, Sydney South 1232 Phone: 131555 (NSW only – publications and information requests) (02) 9995 5000 (switchboard) Fax: (02) 9995 5999 TTY: (02) 9211 4723 Email: [email protected] Website: www.environment.nsw.gov.au DECC 2008/275 ISBN 978 1 74122 839 7 June 2008 Printed on environmentally sustainable paper Cover photos Inset upper: Lake Pinaroo in flood, 1976 (DECC) Aerial: Lake Pinaroo in flood, March 1976 (DECC) Inset lower left: Blue-billed duck (R. Kingsford) Inset lower middle: Red-necked avocet (C. Herbert) Inset lower right: Red-capped plover (C. Herbert) Summary An ecological character description has been defined as ‘the combination of the ecosystem components, processes, benefits and services that characterise a wetland at a given point in time’. -

Adec Preview Generated PDF File
Rec. West. Aust. Mus., 1976,4 (2) THE GENUS MENETIA (LACERTILIA, SCINCIDAE) IN WESTERN AUSTRALIA G.M. STORR* [Received 1 July 1975. Accepted 1 October 1975. Published 30 September 1976.] ABSTRACT The Australian genus Menetia comprises at least five species, three of which occur in Western Australia, namely M. greyii Gray, M. maini novo and M. surda novo A lectotype is designated for M. greyii. INTRODUCTION Until recently all skinks with an immovable transparent lower eyelid were placed in Ablepharus. Fuhn (1969) broke up this polyphyletic assemblage, allotting the Australian species to nine groups, including the genus Menetia. Fuhn, and indeed all workers till now, regarded Menetia as monotypic. Greer (1974) believes that Menetia is derived from the genus Carlia. All the material used in this revision is lodged in the Western Australian Museum. Genus Menetia Gray Menetia Gray, 1845, 'Catalogue of the specimens of lizards in the collection ofthe British Museum', p.65. Type-species (by monotypy): M. greyii Gray. * Curator of Birds and Reptiles, W.A. Museum. 189 Diagnosis Very small, smooth, terrestrial skinks with lower eyelid immovable and bearing a large circular transparent disc incompletely surrounded by granules; digits 4 + 5; first supraocular long and narrow and obliqu~ly orientated. Distribution Most of Australia except the wettest and coolest regions. At least five species, three of them in Western Australia. Description Snout-vent length up to 38 mm. Tail fragile, 1.2-2.0 times as long as snout to vent. Nasals usually separated widely. No supranasals or postnasals. Prefrontals usually separated very narrowly. Frontal small, little if any larger than prefrontals. -

Fowlers Gap Biodiversity Checklist Reptiles
Fowlers Gap Biodiversity Checklist ow if there are so many lizards then they should make tasty N meals for someone. Many of the lizard-eaters come from their Reptiles own kind, especially the snake-like legless lizards and the snakes themselves. The former are completely harmless to people but the latter should be left alone and assumed to be venomous. Even so it odern reptiles are at the most diverse in the tropics and the is quite safe to watch a snake from a distance but some like the Md rylands of the world. The Australian arid zone has some of the Mulga Snake can be curious and this could get a little most diverse reptile communities found anywhere. In and around a disconcerting! single tussock of spinifex in the western deserts you could find 18 species of lizards. Fowlers Gap does not have any spinifex but even he most common lizards that you will encounter are the large so you do not have to go far to see reptiles in the warmer weather. Tand ubiquitous Shingleback and Central Bearded Dragon. The diversity here is as astonishing as anywhere. Imagine finding six They both have a tendency to use roads for passage, warming up or species of geckos ranging from 50-85 mm long, all within the same for display. So please slow your vehicle down and then take evasive genus. Or think about a similar diversity of striped skinks from 45-75 action to spare them from becoming a road casualty. The mm long! How do all these lizards make a living in such a dry and Shingleback is often seen alone but actually is monogamous and seemingly unproductive landscape? pairs for life. -

A Taxonomic Revision of the Genus <I>Menetia</I> (Lacertilia
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Rankin, Peter R., 1979. A taxonomic revision of the genus Menetia (Lacertilia, Scincidae) in the Northern Territory. Records of the Australian Museum 32(14): 491–499. [31 December 1979]. doi:10.3853/j.0067-1975.32.1979.462 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia A TAXONOMIC REVISION OF THE GENUS MENETlA (LACERTILlA, SClNCIDAE) IN THE NORTHERN TERRITORY PETER R. RANKIN*, 12 Finlays Ave., Earlwood N.S.W. ABSTRACT The genus Menetia in the Northern Territory comprises three species: M. alanae sp. nov., M. greyii (Gray), and M. maini Storr. M. zynja Ingram, first described from Queensland is synonymized with M. maini Storr. INTRODUCTION Recently, the genus Menetia has come under scrutiny (Storr, 1976; Ingram, 1977) with the result that a previously monotypic genus now has five species allotted to it. The above studies dealt only with Menetia from Western Australia and Queensland. The present study examines Menetia from the Northern Territory in which there are representatives from both Western Australia and Queensland. Storr (1976) provided a definition of the genus Menetia which was later amended by Ingram (1977), and all species considered here are within the genus as defined by Storr (1976). The term presuboculars is used in the present paper to refer to the scale or scales located in a diagonal line between the posterior lareal and the subocular labial (Fig. -

Brigalow Belt Bioregion – a Biodiversity Jewel
Brigalow Belt bioregion – a biodiversity jewel Brigalow habitat © Craig Eddie What is brigalow? including eucalypt and cypress pine forests and The term ‘brigalow’ is used simultaneously to refer to; woodlands, grasslands and other Acacia dominated the tree Acacia harpophylla; an ecological community ecosystems. dominated by this tree and often found in conjunction with other species such as belah, wilga and false Along the eastern boundary of the Brigalow Belt are sandalwood; and a broader region where this species scattered patches of semi-evergreen vine thickets with and ecological community are present. bright green canopy species that are highly visible among the more silvery brigalow communities. These The Brigalow Belt bioregion patches are a dry adapted form of rainforest, relics of a much wetter past. The Brigalow Belt bioregion is a large and complex area covering 36,400 000ha. The region is thus recognised What are the issues? by the Australian Government as a biodiversity hotspot. Nature conservation in the region has received increasing attention because of the rapid and extensive This hotspot contains some of the most threatened loss of habitat that has occurred. Since World War wildlife in the world, including populations of the II the Brigalow Belt bioregion has become a major endangered bridled nail-tail wallaby and the only agricultural and pastoral area. Broad-scale clearing for remaining wild population of the endangered northern agriculture and unsustainable grazing has fragmented hairy-nosed wombat. The area contains important the original vegetation in the past, particularly on habitat for rare and threatened species including the, lowland areas. glossy black-cockatoo, bulloak jewel butterfl y, brigalow scaly-foot, red goshawk, little pied bat, golden-tailed geckos and threatened community of semi evergreen Biodiversity hotspots are areas that support vine thickets. -
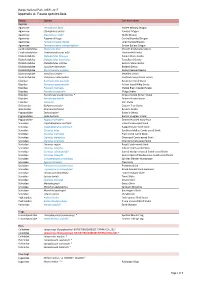
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

Fauna of Australia 2A
FAUNA of AUSTRALIA 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA Mark N. Hutchinson & Stephen C. Donnellan 26. BIOGEOGRAPHY AND PHYLOGENY OF THE SQUAMATA This review summarises the current hypotheses of the origin, antiquity and history of the order Squamata, the dominant living reptile group which comprises the lizards, snakes and worm-lizards. The primary concern here is with the broad relationships and origins of the major taxa rather than with local distributional or phylogenetic patterns within Australia. In our review of the phylogenetic hypotheses, where possible we refer principally to data sets that have been analysed by cladistic methods. Analyses based on anatomical morphological data sets are integrated with the results of karyotypic and biochemical data sets. A persistent theme of this chapter is that for most families there are few cladistically analysed morphological data, and karyotypic or biochemical data sets are limited or unavailable. Biogeographic study, especially historical biogeography, cannot proceed unless both phylogenetic data are available for the taxa and geological data are available for the physical environment. Again, the reader will find that geological data are very uncertain regarding the degree and timing of the isolation of the Australian continent from Asia and Antarctica. In most cases, therefore, conclusions should be regarded very cautiously. The number of squamate families in Australia is low. Five of approximately fifteen lizard families and five or six of eleven snake families occur in the region; amphisbaenians are absent. Opinions vary concerning the actual number of families recognised in the Australian fauna, depending on whether the Pygopodidae are regarded as distinct from the Gekkonidae, and whether sea snakes, Hydrophiidae and Laticaudidae, are recognised as separate from the Elapidae. -

(D. Portschinskii, D. Mixta) Rock Lizards in the Caucasus
mathematics Article Species Distribution Models and Niche Partitioning among Unisexual Darevskia dahli and Its Parental Disexual (D. portschinskii, D. mixta) Rock Lizards in the Caucasus Varos Petrosyan 1,*, Fedor Osipov 1, Vladimir Bobrov 1 , Natalia Dergunova 1, Andrey Omelchenko 1 , Alexander Varshavskiy 1, Felix Danielyan 2 and Marine Arakelyan 2 1 A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow 119071, Russia; [email protected] (F.O.); [email protected] (V.B.); [email protected] (N.D.); [email protected] (A.O.); [email protected] (A.V.) 2 Department of Biology, Yerevan State University, Yerevan 0025, Armenia; [email protected] (F.D.); [email protected] (M.A.) * Correspondence: [email protected] Received: 14 July 2020; Accepted: 6 August 2020; Published: 10 August 2020 Abstract: Among vertebrates, true parthenogenesis is known only in reptiles. Parthenogenetic lizards of the genus Darevskia emerged as a result of the hybridization of bisexual parental species. However, uncertainty remains about the mechanisms of the co-existence of these forms. The geographical parthenogenesis hypothesis suggests that unisexual forms can co-exist with their parental species in the “marginal” habitats. Our goal is to investigate the influence of environmental factors on the formation of ecological niches and the distribution of lizards. For this reason, we created models of species distribution and ecological niches to predict the potential geographical distribution of the parthenogenetic and its parental species. We also estimated the realized niches breadth, their overlap, similarities, and shifts in the entire space of predictor variables. We found that the centroids of the niches of the three studied lizards were located in the mountain forests. -

A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes
BMC Evolutionary Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes BMC Evolutionary Biology 2013, 13:93 doi:10.1186/1471-2148-13-93 Robert Alexander Pyron ([email protected]) Frank T Burbrink ([email protected]) John J Wiens ([email protected]) ISSN 1471-2148 Article type Research article Submission date 30 January 2013 Acceptance date 19 March 2013 Publication date 29 April 2013 Article URL http://www.biomedcentral.com/1471-2148/13/93 Like all articles in BMC journals, this peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in BMC journals are listed in PubMed and archived at PubMed Central. For information about publishing your research in BMC journals or any BioMed Central journal, go to http://www.biomedcentral.com/info/authors/ © 2013 Pyron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes Robert Alexander Pyron 1* * Corresponding author Email: [email protected] Frank T Burbrink 2,3 Email: [email protected] John J Wiens 4 Email: [email protected] 1 Department of Biological Sciences, The George Washington University, 2023 G St. -

A LIST of the VERTEBRATES of SOUTH AUSTRALIA
A LIST of the VERTEBRATES of SOUTH AUSTRALIA updates. for Edition 4th Editors See A.C. Robinson K.D. Casperson Biological Survey and Research Heritage and Biodiversity Division Department for Environment and Heritage, South Australia M.N. Hutchinson South Australian Museum Department of Transport, Urban Planning and the Arts, South Australia 2000 i EDITORS A.C. Robinson & K.D. Casperson, Biological Survey and Research, Biological Survey and Research, Heritage and Biodiversity Division, Department for Environment and Heritage. G.P.O. Box 1047, Adelaide, SA, 5001 M.N. Hutchinson, Curator of Reptiles and Amphibians South Australian Museum, Department of Transport, Urban Planning and the Arts. GPO Box 234, Adelaide, SA 5001updates. for CARTOGRAPHY AND DESIGN Biological Survey & Research, Heritage and Biodiversity Division, Department for Environment and Heritage Edition Department for Environment and Heritage 2000 4thISBN 0 7308 5890 1 First Edition (edited by H.J. Aslin) published 1985 Second Edition (edited by C.H.S. Watts) published 1990 Third Edition (edited bySee A.C. Robinson, M.N. Hutchinson, and K.D. Casperson) published 2000 Cover Photograph: Clockwise:- Western Pygmy Possum, Cercartetus concinnus (Photo A. Robinson), Smooth Knob-tailed Gecko, Nephrurus levis (Photo A. Robinson), Painted Frog, Neobatrachus pictus (Photo A. Robinson), Desert Goby, Chlamydogobius eremius (Photo N. Armstrong),Osprey, Pandion haliaetus (Photo A. Robinson) ii _______________________________________________________________________________________ CONTENTS -

Supplementary Methods S1
1 Validation methods for trophic niche models 2 3 To assign links between nodes (species), we used trophic niche-space models (e.g., [1]). 4 Each of these models has two quantile regressions that define the prey-size range a 5 predator of a given size is predicted to consume. Species whose body mass is within the 6 range of a predator’s prey size, as identified by the trophic niche-space model, are predicted 7 to be prey, while those outside the range are predicted not to be eaten. 8 9 The broad taxonomy of a predator helps to predict predation interactions [2]. To optimize 10 our trophic niche-space model, we therefore tested whether including taxonomic class of 11 predators improved the fit of quantile regressions. Using trophic (to identify which species 12 were predators), body mass, and taxonomic data, we fitted and compared five quantile 13 regression models (including a null model) to the GloBI data. In each model, we log10- 14 transformed the dependent variable prey body mass, and included for the independent 15 variables different combinations of log10-transformed predator body mass, predator class, 16 and the interaction between these variables (Supplementary Table S4). We log10- 17 transformed both predator and prey body mass to linearize the relationship between these 18 variables. We fit the five quantile regressions to the upper and lower 5% of prey body mass, 19 and compared model fits using the Bayesian information criterion (BIC). The predator body 20 mass*predator class model fit the 95th quantile data best, whereas the predator body mass 21 + predator class model fit the 5th quantile data marginally better than the aforementioned 22 interaction model (Supplementary Figure S2, Supplementary Table S4). -

Species Richness in Time and Space: a Phylogenetic and Geographic Perspective
Species Richness in Time and Space: a Phylogenetic and Geographic Perspective by Pascal Olivier Title A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Ecology and Evolutionary Biology) in The University of Michigan 2018 Doctoral Committee: Assistant Professor and Assistant Curator Daniel Rabosky, Chair Associate Professor Johannes Foufopoulos Professor L. Lacey Knowles Assistant Professor Stephen A. Smith Pascal O Title [email protected] ORCID iD: 0000-0002-6316-0736 c Pascal O Title 2018 DEDICATION To Judge Julius Title, for always encouraging me to be inquisitive. ii ACKNOWLEDGEMENTS The research presented in this dissertation has been supported by a number of research grants from the University of Michigan and from academic societies. I thank the Society of Systematic Biologists, the Society for the Study of Evolution, and the Herpetologists League for supporting my work. I am also extremely grateful to the Rackham Graduate School, the University of Michigan Museum of Zoology C.F. Walker and Hinsdale scholarships, as well as to the Department of Ecology and Evolutionary Biology Block grants, for generously providing support throughout my PhD. Much of this research was also made possible by a Rackham Predoctoral Fellowship, and by a fellowship from the Michigan Institute for Computational Discovery and Engineering. First and foremost, I would like to thank my advisor, Dr. Dan Rabosky, for taking me on as one of his first graduate students. I have learned a tremendous amount under his guidance, and conducting research with him has been both exhilarating and inspiring. I am also grateful for his friendship and company, both in Ann Arbor and especially in the field, which have produced experiences that I will never forget.