Morphological Complexity As a Floral Signal: from Perception by Insect Pollinators to Co-Evolutionary Implications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annotated Checklist of Vascular Flora, Bryce
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 ON THE COVER Matted prickly-phlox (Leptodactylon caespitosum), Bryce Canyon National Park, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Sarah Topp Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 January 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Polemonium Viscosum Nutt
Polemonium viscosum Nutt. skunk polemonium Polemoniaceae - phlox family status: State Sensitive, BLM sensitive, USFS sensitive rank: G5 / S1S2 General Description: Low perennial from a stout taproot and branched caudex, up to 2 (4) dm in height, densely glandular or hairy-glandular, with a strong skunky odor. Leaves pinnately compound, mostly basal, up to 1.5 (2) dm long, including the short petiole. Leaflets numerous, crowded, opposite, with usually 2-5 lobes cleft almost to the base, thus appearing whorled. The individual lobes are rounded, 1.5-6 mm x 1-3 mm. Floral Characteristics: Inflorescence dense, headlike, usually elongating a little in fruit. C alyx tubular, glandular, 7-12 mm long when flowering; lobes 5, narrow, pointed, and shorter than the tube. C orolla blue, funnel or tubular-funnel shaped, often conspicuously longer than wide, (13) 17-25 (30) mm long, with 5 lobes that are shorter than the tube. Flowers July to A ugust. Fruits: C aps ules . Illustration by Jeanne R. Janish, ©1959 University of Washington Press Identif ication Tips: Polemonium elegans is also strongly scented and has a corolla longer than wide. However, P. elegans is a much shorter plant (up to 15 cm tall) with a smaller corolla (12-15 mm long) and entire leaflets that do not appear whorled. Range: C oast ranges of southern B.C . to southwest A lberta, south to A Z and NM; absent from CA . Habitat/Ecology: A t high altitudes, commonly above timberline, in open rocky places, talus slopes, rock outcrops, glacial cirques, and alpine fellfields. Elevations in WA: 1900-2500 m (6350-8200 ft). -

Reproductive Biology of Wild Blueberry (Vaccinium Angustifolium Aiton)
agriculture Article Reproductive Biology of Wild Blueberry (Vaccinium angustifolium Aiton) Frank Drummond School of Biology and Ecology, University of Maine, Orono, ME 04469, USA; [email protected]; Tel.: +1-(207)-581-2989 Received: 7 March 2019; Accepted: 25 March 2019; Published: 30 March 2019 Abstract: Wild blueberry, Vaccinium angustifolium Aiton, is a native forest understory plant that is managed as a fruit crop. Over the past 51 years, experiments have been conducted to investigate its reproduction. A model was developed that predicts bloom to begin at 100◦ days (base 4.4 ◦C) after 1 April and to end at 500◦ days for a period of three to four weeks. Flower stigmas are only receptive to pollen deposition for eight to 10 days, and the rate of fruit set declines rapidly after four days. Placement of pollen upon receptive stigmas suggests that fruit set occurs with as little as a single pollen tetrad. Twelve tetrads result in 50% fruit set. Several years of exploratory fruit set field experiments show viable seeds per berry, which result from pollination with compatible genotype pollen, is associated with larger berry mass (g). Decomposition of the total variance in fruit set shows that stem variation explains 65% to 79% of total variance in the fruit set. To a lesser extent, the field, year, and clone also explain the percent fruit set variation. Variation between stems may be due to variation in the number of flowers. Fruit set tends to decrease as the flower density increases, possibly due to the limitation of pollinators. Keywords: hand pollination; stigma viability; phenology; fruit set; bloom prediction 1. -

Draft Columbia Cascade Ecoprovince Wildlife Assessment and Inventory
Draft Columbia Cascade Ecoprovince Wildlife Assessment and Inventory Submitted by Paul Ashley and Stacey H. Stovall Table of Contents Table of Contents........................................................................................................................... i List of Figures .............................................................................................................................. iii List of Tables................................................................................................................................. v 1.0 Wildlife Assessment Framework .......................................................................................1 1.1 Assessment Tools.......................................................................................................3 2.0 Physical Features..............................................................................................................3 2.1 Land Area....................................................................................................................3 2.2 Physiography...............................................................................................................4 3.0 Socio-Political Features ....................................................................................................5 3.1 Land Ownership ..........................................................................................................5 3.2 Land Use.....................................................................................................................7 -
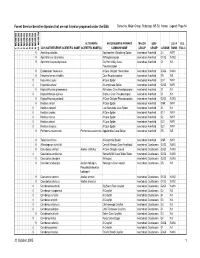
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

The Good, the Bad, and the Ugly: Pollinators As Vectors of Mummy Berry Disease in Highbush Blueberry
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations Dissertations and Theses March 2019 The Good, The Bad, and The Ugly: Pollinators as Vectors of Mummy Berry Disease in Highbush Blueberry Matthew Boyer University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_2 Part of the Agricultural Science Commons, Agronomy and Crop Sciences Commons, Apiculture Commons, Entomology Commons, Fruit Science Commons, Other Ecology and Evolutionary Biology Commons, Plant Biology Commons, and the Plant Pathology Commons Recommended Citation Boyer, Matthew, "The Good, The Bad, and The Ugly: Pollinators as Vectors of Mummy Berry Disease in Highbush Blueberry" (2019). Doctoral Dissertations. 1473. https://doi.org/10.7275/96t5-ar91 https://scholarworks.umass.edu/dissertations_2/1473 This Open Access Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. THE GOOD, THE BAD, AND THE UGLY: POLLINATORS AS VECTORS OF MUMMY BERRY DISEASE IN HIGHBUSH BLUEBERRY A Dissertation Presented by MATTHEW D. H. BOYER Submitted to the Graduate School of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY February 2019 Organismic and Evolutionary Biology and Entomology -

Chapter 4. Butterflies in Many Patches
Assessing the conservation status of the Sinai Baton Blue butterfly (Pseudophilotes sinaicus) Katy Thompson, BSc. (Hons) Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy July 2013 Abstract Arid environments are resource-limited, with scarcity of water the key limiting factor for plants and their associated fauna. Consequentially bottom-up forces often control food webs, influencing the whole system through high levels of competition. The Sinai Baton Blue butterfly, Pseudophilotes sinaicus, is Critically Endangered, with a tiny endemic distribution in the St Katherine Protectorate, South Sinai, an arid environment. Its range is restricted to that of its sole host plant, the near- endemic endangered Sinai Thyme, Thymus decussatus, leaving the butterfly in a highly fragmented distribution. This study looks into the spatio-temporal variations in quality and abundance of the host plant and its implications for the Sinai Baton Blue. Over the past decade the butterfly has exhibited severe population cycles, with the causes still unclear; it could be due to the fluctuating resource levels with large temporal variation in the quality of thyme and density of inflorescences. The number of flowers significantly influences the larval distribution, indicating that resources play a key role in offspring survivorship. Population viability analysis has also highlighted the importance of management techniques aimed at increasing the butterfly's survivorship. The butterfly population sizes are positively correlated with the total resource area and the number of host plants but not the distance between habitat patches. Population viability analysis also suggests that habitat area is more influential than connectivity in this system driving current dynamics. -

Fall 2006 Kelseya
Fall 2006 Kelseya Volume 20 No. 1 e i n Kelseya n o B : n Newsletter of the Montana Native Plant Society o i t a r t s www.umt.edu/mnps/ u l l I Natural Prairie Holds Key to Sustainable Fuels gas prices inch higher, produce concentrated energy in the make and apply. Experiments now the search is on for re- form of biofuels such as ethanol or under way in Germany and the Neth- As newable, plant-based synfuel gasoline and diesel. The erlands are noting similar effects of fuels that don't require fertilizer or greater the yield of biomass per acre diversity on yields, says Tilman, even pesticides, both of which require en- the better, and data from Cedar though they use totally different spe- ergy to produce. A solution may be at Creek show that diverse plantings fill cies. Also, because prairie plants are hand, from University of Minnesota the bill. perennial, they would not have to be ecologist David Tilman and two col- "Diverse prairie grasslands are 240 replanted year after year. Farmers leagues: Instead of growing a single percent more productive than grass- would need only mow their fields in fuel source crop, grow many species lands with a single prairie species," the fall. If burned, biomass could together because such plantations says Tilman, a Regents Professor of replace some of the coal that now will yield more total vegetation—and Ecology in the College of Biological pumps carbon dioxide and mercury do it more reliably—than any growing Sciences, which operates the Cedar into the atmosphere. -

FERNS and FERN ALLIES Dittmer, H.J., E.F
FERNS AND FERN ALLIES Dittmer, H.J., E.F. Castetter, & O.M. Clark. 1954. The ferns and fern allies of New Mexico. Univ. New Mexico Publ. Biol. No. 6. Family ASPLENIACEAE [1/5/5] Asplenium spleenwort Bennert, W. & G. Fischer. 1993. Biosystematics and evolution of the Asplenium trichomanes complex. Webbia 48:743-760. Wagner, W.H. Jr., R.C. Moran, C.R. Werth. 1993. Aspleniaceae, pp. 228-245. IN: Flora of North America, vol.2. Oxford Univ. Press. palmeri Maxon [M&H; Wagner & Moran 1993] Palmer’s spleenwort platyneuron (Linnaeus) Britton, Sterns, & Poggenburg [M&H; Wagner & Moran 1993] ebony spleenwort resiliens Kunze [M&H; W&S; Wagner & Moran 1993] black-stem spleenwort septentrionale (Linnaeus) Hoffmann [M&H; W&S; Wagner & Moran 1993] forked spleenwort trichomanes Linnaeus [Bennert & Fischer 1993; M&H; W&S; Wagner & Moran 1993] maidenhair spleenwort Family AZOLLACEAE [1/1/1] Azolla mosquito-fern Lumpkin, T.A. 1993. Azollaceae, pp. 338-342. IN: Flora of North America, vol. 2. Oxford Univ. Press. caroliniana Willdenow : Reports in W&S apparently belong to Azolla mexicana Presl, though Azolla caroliniana is known adjacent to NM near the Texas State line [Lumpkin 1993]. mexicana Schlechtendal & Chamisso ex K. Presl [Lumpkin 1993; M&H] Mexican mosquito-fern Family DENNSTAEDTIACEAE [1/1/1] Pteridium bracken-fern Jacobs, C.A. & J.H. Peck. Pteridium, pp. 201-203. IN: Flora of North America, vol. 2. Oxford Univ. Press. aquilinum (Linnaeus) Kuhn var. pubescens Underwood [Jacobs & Peck 1993; M&H; W&S] bracken-fern Family DRYOPTERIDACEAE [6/13/13] Athyrium lady-fern Kato, M. 1993. Athyrium, pp. -

Hydraulic Conductance and the Maintenance of Water Balance in Flowers
Plant, Cell and Environment (2016) 39,2123–2132 doi: 10.1111/pce.12761 Original Article Hydraulic conductance and the maintenance of water balance in flowers Adam B. Roddy1,2, Craig R. Brodersen2 & Todd E. Dawson1 1Department of Integrative Biology, University of California, Berkeley, CA 94720, USA and 2School of Forestry & Environmental Studies, Yale University, New Haven, CT 06511, USA ABSTRACT been relatively ignored, even though non-pollinator agents of selection, such as physiological costs, often oppose the effects Flowers face desiccating conditions, yet little is known about of pollinators (Galen 1999; Galen et al. 1999; Lambrecht their ability to transport water. We quantified variability in 2013; Strauss and Whittall 2006). floral hydraulic conductance (Kfl )for20speciesfrom10 ower Like leaves, flowers are terminal structures often located in families and related it to traits hypothesized to be associated the hottest, driest parts of the plant canopy, but flowers and with liquid and vapour phase water transport. Basal angio- leaves have experienced different selective forces. Leaves are sperm flowers had trait values associated with higher water a critical component in the plant hydraulic pathway and have and carbon costs than monocot and eudicot flowers. Kfl ower alargeinfluence on the terrestrial water cycle (Hetherington was coordinated with water supply (vein length per area, and Woodward 2003). Because carbon assimilation is mecha- VLA) and loss (minimum epidermal conductance, g ) traits min nistically linked to plant transpiration (Brodribb and Feild among the magnoliids, but was insensitive to variation in these 2000; Brodribb et al. 2007; Sack and Holbrook 2006), moving traits among the monocots and eudicots. Phylogenetic inde- water from the roots to the leaves requires coordination in pendent contrast (PIC) correlations revealed that few traits the structural traits governing water flow through each compo- had undergone coordinated evolution. -

Floral Volatiles Controlling Ant Behaviour
Functional Ecology 2009, 23, 888–900 doi: 10.1111/j.1365-2435.2009.01632.x FLORAL SCENT IN A WHOLE-PLANT CONTEXT Floral volatiles controlling ant behaviour Pat G. Willmer*,1, Clive V. Nuttman1, Nigel E. Raine2, Graham N. Stone3, Jonathan G. Pattrick1, Kate Henson1, Philip Stillman1, Lynn McIlroy1, Simon G. Potts4 and Jeffe T. Knudsen5 1School of Biology, University of St Andrews, Fife KY16 9TS, Scotland, UK; 2Research Centre for Psychology, School of Biological & Chemical Sciences, Queen Mary University of London, Mile End Road, London, E1 4NS, UK; 3Institute of Evolutionary Biology, School of Biology, University of Edinburgh, Kings Buildings, Edinburgh EH9 3JT, Scotland, UK; 4Centre for Agri-Environmental Research, University of Reading, Reading, RG6 6AR, UK; and 5Department of Ecology, Lund University, Solvegatan 37, SE-223 62 Lund, Sweden Summary 1. Ants show complex interactions with plants, both facultative and mutualistic, ranging from grazers through seed predators and dispersers to herders of some herbivores and guards against others. But ants are rarely pollinators, and their visits to flowers may be detrimental to plant fitness. 2. Plants therefore have various strategies to control ant distributions, and restrict them to foliage rather than flowers. These ‘filters’ may involve physical barriers on or around flowers, or ‘decoys and bribes’ sited on the foliage (usually extrafloral nectaries - EFNs). Alternatively, volatile organic compounds (VOCs) are used as signals to control ant behaviour, attracting ants to leaves and ⁄ or deterring them from functional flowers. Some of the past evidence that flowers repel ants by VOCs has been equivocal and we describe the shortcomings of some experimental approaches, which involve behavioural tests in artificial conditions.