1 Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nervous Tissue
Nervous Tissue Prof.Prof. ZhouZhou LiLi Dept.Dept. ofof HistologyHistology andand EmbryologyEmbryology Organization:Organization: neuronsneurons (nerve(nerve cells)cells) neuroglialneuroglial cellscells Function:Function: Ⅰ Neurons 1.1. structurestructure ofof neuronneuron somasoma neuriteneurite a.a. dendritedendrite b.b. axonaxon 1.11.1 somasoma (1)(1) nucleusnucleus LocatedLocated inin thethe centercenter ofof soma,soma, largelarge andand palepale--stainingstaining nucleusnucleus ProminentProminent nucleolusnucleolus (2)(2) cytoplasmcytoplasm (perikaryon)(perikaryon) a.a. NisslNissl bodybody b.b. neurofibrilneurofibril NisslNissl’’ss bodiesbodies LM:LM: basophilicbasophilic massmass oror granulesgranules Nissl’s Body (TEM) EMEM:: RERRER,, freefree RbRb FunctionFunction:: producingproducing thethe proteinprotein ofof neuronneuron structurestructure andand enzymeenzyme producingproducing thethe neurotransmitterneurotransmitter NeurofibrilNeurofibril thethe structurestructure LM:LM: EM:EM: NeurofilamentNeurofilament micmicrotubulerotubule FunctionFunction cytoskeleton,cytoskeleton, toto participateparticipate inin substancesubstance transporttransport LipofuscinLipofuscin (3)(3) CellCell membranemembrane excitableexcitable membranemembrane ,, receivingreceiving stimutation,stimutation, fromingfroming andand conductingconducting nervenerve impulesimpules neurite: 1.2 Dendrite dendritic spine spine apparatus Function: 1.3 Axon axon hillock, axon terminal, axolemma Axoplasm: microfilament, microtubules, neurofilament, mitochondria, -

Sensory Pathways and the Somatic Nervous System
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint® Lecture Presentations prepared by Jason LaPres Lone Star College—North Harris © 2012 Pearson Education, Inc. 15-1 Sensory Information • Afferent Division of the Nervous System • Receptors • Sensory neurons • Sensory pathways • Efferent Division of the Nervous System • Nuclei • Motor tracts • Motor neurons © 2012 Pearson Education, Inc. Figure 15-1 An Overview of Neural Integration OVERVIEW OF NEURAL INTEGRATION CHAPTER 15 CHAPTER 16 Sensory Higher-Order Functions processing Conscious and Memory, learning, and centers in subconscious intelligence may brain motor centers in brain influence interpretation of sensory information and nature of motor activities Sensory Motor pathways pathways Somatic Autonomic Nervous Nervous System (SNS) System (ANS) General Skeletal Visceral effectors sensory muscles (examples: smooth receptors muscles, glands, cardiac muscle, adipocytes) © 2012 Pearson Education, Inc. 15-1 Sensory Information • Sensory Receptors • Specialized cells that monitor specific conditions • In the body or external environment • When stimulated, a receptor passes information to the CNS • In the form of action potentials along the axon of a sensory neuron © 2012 Pearson Education, Inc. 15-1 Sensory Information • Sensory Pathways • Deliver somatic and visceral sensory information to their final destinations inside the CNS using: • Nerves • Nuclei • Tracts © 2012 Pearson Education, Inc. 15-1 Sensory Information • Somatic Motor Portion of the Efferent Division • Controls peripheral effectors • Somatic Motor Commands • Travel from motor centers in the brain along somatic motor pathways of: • Motor nuclei • Tracts • Nerves © 2012 Pearson Education, Inc. 15-1 Sensory Information • Somatic Nervous System (SNS) • Motor neurons and pathways that control skeletal muscles © 2012 Pearson Education, Inc. -

Sensory Dysfunction in Children with Tourette Syndrome
Sensory Dysfunction in Children with Tourette Syndrome by Nasrin Shahana, MBBS A Dissertation Submitted inPartial Fulfillment of theRequirements for the Degree ofDoctor of Philosophyin Neuroscience University of Cincinnati Cincinnati Children’s Hospital 12th August 2015 Committee Members James Eliassen, PhD (Chair) Matthew R. Skelton, PhD Michael P. Jankowski, PhD Jennifer J. Vannest, PhD Donald L. Gilbert, MS, MD (Advisor) 1 ABSTRACT Sensory Dysfunction in Children with Tourette Syndrome By Nasrin Shahana, MBBS The University of Cincinnati, 2015 Under the Supervision of Donald L. Gilbert, MS, MD Prime symptoms of Tourette Syndrome (TS) constitute motor and vocal tics but observation from clinical standpoints as well as parents or individual patient’s observation has provided evidence for sensory abnormalities associated with TS. One of the well-known phenomenon is tic related premonitory urges (PU’s), described as recurring, intrusive sensory feelings such as discomfort, pressure etc. that precede and in some cases compel performance of tics. Sensory sensitivity or intolerance is often reported to be related to being sensitive to the external sensory information such as intolerance to clothing tags. Some report urges to have subconsciously copying movements (echopraxia) or speech (echolalia) of others. Patients often complain about their tics being enhanced by certain sensory stimuli like sounds or lights. With the exception of Premonitory Urges (PU’s), most of the sensory symptoms have not been addressed by standard clinical practice. PU’s can be evaluated with a standard clinical rating scale called Premonitory Urges in Tourette Syndrome (PUTS) which tends to be well correlated with tics. PU’s seem to be a critical distinguishing factor between TS and other movement disorders. -

Sensory Receptors A17 (1)
SENSORY RECEPTORS A17 (1) Sensory Receptors Last updated: April 20, 2019 Sensory receptors - transducers that convert various forms of energy in environment into action potentials in neurons. sensory receptors may be: a) neurons (distal tip of peripheral axon of sensory neuron) – e.g. in skin receptors. b) specialized cells (that release neurotransmitter and generate action potentials in neurons) – e.g. in complex sense organs (vision, hearing, equilibrium, taste). sensory receptor is often associated with nonneural cells that surround it, forming SENSE ORGAN. to stimulate receptor, stimulus must first pass through intervening tissues (stimulus accession). each receptor is adapted to respond to one particular form of energy at much lower threshold than other receptors respond to this form of energy. adequate (s. appropriate) stimulus - form of energy to which receptor is most sensitive; receptors also can respond to other energy forms, but at much higher thresholds (e.g. adequate stimulus for eye is light; eyeball rubbing will stimulate rods and cones to produce light sensation, but threshold is much higher than in skin pressure receptors). when information about stimulus reaches CNS, it produces: a) reflex response b) conscious sensation c) behavior alteration SENSORY MODALITIES Sensory Modality Receptor Sense Organ CONSCIOUS SENSATIONS Vision Rods & cones Eye Hearing Hair cells Ear (organ of Corti) Smell Olfactory neurons Olfactory mucous membrane Taste Taste receptor cells Taste bud Rotational acceleration Hair cells Ear (semicircular -

Sensory Pathways and the Somatic Nervous System
Fundamentals of Anatomy & Physiology Eleventh Edition Chapter 15 Sensory Pathways and the Somatic Nervous System Lecture Presentation by Lori Garrett, Parkland College © 2018 Pearson Education, Inc. Learning Outcomes 15-1 Specify the components of the afferent and efferent divisions of the nervous system, and explain what is meant by the somatic nervous system. 15-2 Explain why receptors respond to specific stimuli, and how the organization of a receptor affects its sensitivity. 15-3 Identify the receptors for the general senses, and describe how they function. 15-4 Identify the major sensory pathways, and explain how it is possible to distinguish among sensations that originate in different areas of the body. 15-5 Describe the components, processes, and functions of the somatic motor pathways, and the levels of information processing involved in motor control. 2 © 2018 Pearson Education, Inc. Sensory Pathways and Somatic Nervous System ▪ Focus for this chapter – Sensory pathways • General senses – Motor pathways • Somatic nervous system (SNS) – Controls contractions of skeletal muscles 3 © 2018 Pearson Education, Inc. 15-1 Sensory and Motor Pathways ▪ Sensory pathways – Series of neurons that relays sensory information from receptors to CNS ▪ Sensory receptors – Specialized cells or cell processes that monitor specific conditions • In the body or external environment – When stimulated, a receptor generates action potentials that are sent along sensory pathways 4 © 2018 Pearson Education, Inc. 15-1 Sensory and Motor Pathways ▪ Afferent division of nervous system – Somatic and visceral sensory pathways ▪ Efferent division of nervous system – Somatic motor portion • Carries out somatic motor commands that control peripheral effectors • Commands travel from motor centers in brain along somatic motor pathways 5 © 2018 Pearson Education, Inc. -

The Nervous System: General and Special Senses
18 The Nervous System: General and Special Senses PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • Sensory information arrives at the CNS • Information is “picked up” by sensory receptors • Sensory receptors are the interface between the nervous system and the internal and external environment • General senses • Refers to temperature, pain, touch, pressure, vibration, and proprioception • Special senses • Refers to smell, taste, balance, hearing, and vision © 2012 Pearson Education, Inc. Receptors • Receptors and Receptive Fields • Free nerve endings are the simplest receptors • These respond to a variety of stimuli • Receptors of the retina (for example) are very specific and only respond to light • Receptive fields • Large receptive fields have receptors spread far apart, which makes it difficult to localize a stimulus • Small receptive fields have receptors close together, which makes it easy to localize a stimulus. © 2012 Pearson Education, Inc. Figure 18.1 Receptors and Receptive Fields Receptive Receptive field 1 field 2 Receptive fields © 2012 Pearson Education, Inc. Receptors • Interpretation of Sensory Information • Information is relayed from the receptor to a specific neuron in the CNS • The connection between a receptor and a neuron is called a labeled line • Each labeled line transmits its own specific sensation © 2012 Pearson Education, Inc. Interpretation of Sensory Information • Classification of Receptors • Tonic receptors -

17 General Senses
17 General Senses UNIT OUTLINE General Sensory Receptors Activity 1: Identifying General Sensory Receptors Activity 2: Examining the Microscopic Structure of General Sensory Receptors Activity 3: Performing a Two-Point Discrimination Test n this unit we investigate sensory receptors, structures that respond to changes, or stimuli, in the external environment and within the body. Most sensory re- Iceptors—those that respond to touch, pressure, cold, heat, pain, stretch, vibra- tion, and changes in body position—are widely distributed throughout the body and are classified as general sensory receptors. Somatic sensory receptors are found in the skin and in musculoskeletal organs, whereas visceral sensory recep- tors are located in visceral organs. Sensory receptors for the special senses, by con- trast, are housed in specialized sense organs in the head. The special senses, which include gustation (taste), olfaction (smell), vision, hearing, and equilibrium, will be our focus in Unit 18. THINK ABOUT IT Which brain structure serves as a relay station for most sensory impulses? __________________________________________________________________ Sensory impulses originating from general sensory receptors in the skin are relayed to which functional area of the cerebral cortex? _______________________________ What is the difference between sensation and perception and where does each occur? __________________________________________________________________ _________________________________________________________________ ▪ 355 PRE-LAB ASSIGNMENTS Pre-lab quizzes are also assignable in To maximize learning, BEFORE your lab period carefully read this entire lab unit and complete these pre-lab assignments using your textbook, lecture notes, and prior knowledge. PRE-LAB Activity 1: Identifying General Sensory Receptors 1. Use the list of terms provided to label the accompanying illustration of cutaneous receptors. -
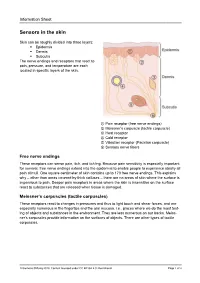
Sensors in the Skin
Information Sheet Sensors in the skin Skin can be roughly divided into three layers: . Epidermis . Dermis . Subcutis The nerve endings and receptors that react to pain, pressure, and temperature are each located in specific layers of the skin. ① Pain receptor (free nerve endings) ② Meissner’s corpuscle (tactile corpuscle) ③ Heat receptor ④ Cold receptor ⑤ Vibration receptor (Pacinian corpuscle) ⑥ Sensory nerve fibers Free nerve endings These receptors can sense pain, itch, and tickling. Because pain sensitivity is especially important for survival, free nerve endings extend into the epidermis to enable people to experience ideally all pain stimuli. One square centimeter of skin contains up to 170 free nerve endings. This explains why – other than areas covered by thick calluses – there are no areas of skin where the surface is impervious to pain. Deeper pain receptors in areas where the skin is insensitive on the surface react to substances that are released when tissue is damaged. Meissner’s corpuscles (tactile corpuscles) These receptors react to changes in pressures and thus to light touch and shear forces, and are especially numerous in the fingertips and the oral mucosa, i.e., places where we do the most test- ing of objects and substances in the environment. They are less numerous on our backs. Meiss- ner’s corpuscles provide information on the surfaces of objects. There are other types of tactile corpuscles. © Siemens Stiftung 2019. Content licensed under CC BY-SA 4.0 international Page 1 of 2 Information Sheet Heat receptors These receptors are located in the dermis. They react to a rise in temperature and are thus in- volved in sensing heat. -
Nervous Tissue Consists of 2 Types of Cells
Nervous Tissue consists of 2 types of cells • 1 - Neurons – main cells (basic functional units), specialized to • perception of sensory stimuli, • processing received information and • transmission it further to other neurons in form of nerve impulses • 2 - Neuroglia-(glial cells) (supporting cells) • they support, • nourish and • protect neurons Neuron Structure 1. Cell body = perikaryon = contains nucleus and is the metabolic center of the cell 2. Processes – that extend from the cell body (dendrites and axon) 3. Nerve endings (synapses, special receptors) Neuron Cell body has: Nucleus with large nucleolus Neurofibrils “Nissl bodies” (chromophilic substance) Neurofibrils are present in the perikaryon, dendrites and axon and are unique to neurons. = “Skeleton” of the neurons Nissl bodies - large clumps of basophilic material around the nucleus, an aggregation of many parallel cisternae of the rough endoplasmic reticulum with the rosettes of free polisomal ribosomes Function – protein synthesis (neurotransmitters) Neuron processes - Extensions outside the cell body Dendrites – conduct impulses toward the cell body Axons – conduct impulses away from the cell body (usually only 1!) All processes end with the nerve endings Slide 8 • Axons are covered with a fatty material called myelin. • Axons in the PNS are heavily myelinated. • This is done by the Schwann Cells • These Schwann cells layer around the axions and squeeze their cytoplasm out creating many layers of plasma membrane tissues (proteins/lipids) surrounding the axion. This is the Myelin sheath. • Areas of neuron not covered are called Nodes of Ranvier. • Myelin insulates the nerve fibers and greatly increases the speed of neurotransmission by nerve fibers. 12-9 • Each axon terminal (synaptic knob) is seperated from the cell body or dendrites of the next neuron by a tiny gap…synaptic cleft. -
A&P Spinal PNS C
Number: CN X Name: Vagus Nerve Fxn: Somatic sensory. Proprioception from pharynx, larynx, ear pinna Somatic motor. Pharyngeal and laryngeal muscles Visceral sensory. Receives input from thoracoabdominal organs Visceral motor. To thoracoabdominal organs Number: CN XI Name: Accessory Nerve Fxn: Somatic motor. Pharyngeal, laryngeal and soft palate muscles. Trapezius. Sternocleidomastoid. Number: CN XII Name: Hypoglossal Nerve Fxn: Somatic motor. Intrinsic & extrinsic muscles of tongue RECEPTORS Receptors are skin and muscle that are specialized to detect and respond to stimuli. They carry impulses to the CNS. They are classified by: o Type of stimulus detected o Location in body o Structural complexity RECEPTORS CLASSIFIED BY TYPE OF STIMULUS DETECTED Mechanoreceptor Response to mechanical distortion Touch, vibration, pressure, itch, stretch Thermoreceptor Changes in temperature Photoreceptor Responds to light energy Chemoreceptors Responds to chemical in solution taste and smell Nocireceptors Responds to potentially damaging stimuli RECEPTORS CLASSIFIED BY WHERE LOCATED IN THE BODY Exteroceptor Sense external stimuli. Includes most special senses Touch, pressure, pain, skin temp Interoceptor Responds to internal stimuli Chemical changes, tissue stretch, temperature, pain Proprioceptor Sense stretch in skeletal muscles, ligaments, tendons, joints, bones and muscle CT coverings RECEPTORS CLASSIFIED BY COMPLEXITY Simple receptors Modified dendritic endings provide most of the general senses Complex receptors Highly specialized sense organs Provide special senses (vision, hearing, equilibrium, taste, smell) MORE DETAIL ABOUT SIMPLE RECEPTORS Simple receptors are either free nerve endings or encapsulated nerve endings. The FREE NERVE ENDING RECEPTORS are typically pain or temperature receptors. They are located especially in epithelium and CT. Some are associated with specific structures. MERKEL DISCS detect light touch and are associated with Merkel cells. -
Nervous Tissue Lec
Nervous Tissue Lec. 10 Second year Histology A.T. Hadeel Kamil 1 Nervous Tissue consists of 2 types of cells 1 - Neurons – main cells, specialized to – perception of sensory stimuli, – processing received information and – transmission it further to other neurons in form of nerve impulses 2 - Neuroglia – they support, – nourish and – protect neurons Neuron Structure 1. Cell body = perikaryon = contains nucleus and is the metabolic center of the cell 2. Processes – that extend from the cell body (dendrites and axon) 3. Nerve endings (synapses, special receptors) 2 Cell body has: 1. Nucleus with large nucleolus 2. Neurofibrils 3. “Nissl bodies” (chromophilic substance) Neurofibrils are present in the perikaryon, dendrites and axon and are unique to neurons. = “Skeleton” of the neurons Nissl bodies • large clumps of basophilic material around the nucleus, an aggregation of many parallel cisternae of the rough endoplasmic reticulum with the rosettes of free polisomal ribosomes Function – protein synthesis (neurotransmitters) 3 Neuron processes - Extensions outside the cell body • Dendrites – conduct impulses toward the cell body • Axons – conduct impulses away from the cell body (usually only 1) • All processes end with the nerve endings 4 Slide 8 Structural Classification of Neurons According to amount of processes 1. Unipolar neurons – are found during early embryogenesis. They have one axon 2. Bipolar neurons – one axon and one dendrite 3. Pseudounipolar neurons – have a short single process leaving the cell body 4. Multipolar neurons – many extensions from the cell body 5 Functional Classification of Neurons 1. Sensory (afferent) neurons Carry impulses from the sensory receptors to the cell body 2. Motor (efferent) neurons Carry impulses from cell body which lie in the central nervous system to effector cells 3. -
Sensory Pathways & Somatic Nervous System
Sensory Pathways & Somatic Nervous System Chapter 15 How Does Brain Differentiate Sensations? Pain impulses make brain aware of injuries and infections. Impulses from eye, ear, nose and tongue make brain aware of what you saw, heard, smelled or tasted. Impulses from joints and ear make brain aware of body position and balance. How does brain differentiate between different impulses/sensations? Depends on which part of the brain these impulses reach. Examples: Occipital lobe translates impulses as light. Temporal lobe translates impulses as sound. Cerebellum translates impulses as equilibrium and balance. General Senses vs. Special Senses There are sensory receptors present all over the body to make brain aware of its environment…sensation! All body senses can be divided into two categories: General senses Special senses General Senses vs. Special Senses Epidermal cells of skin Epidermal cells of skin Dendrites Free nerve endings Sensory nerve fiber Primary sensory Tactile receptors on skin cortex General senses: Monitor simpler, general outside/inside environment. Examples: sense of touch, pressure, temperature, pain and body position. Receptors for general senses: Widely distributed throughout the body… simple receptors. Are dendrites of sensory neurons….which could be naked (free nerve endings) or encapsulated (Meissner’s corpuscle/ Tactile corpuscle). Integrated by primary sensory cortex in the parietal lobe of cerebrum. General Senses vs. Special Senses Special senses: Monitor more complex senses. Examples: sense of vision (sight), auditory (hearing), olfaction (smell), gustation (taste) and equilibrium (balance). Receptors for special senses: Are specialized cells (neurons or epithelial cells). Are found in clusters and localized only in certain parts of the body… complex receptors.