Somatosensory Evoked Potentials Indexing Lateral Inhibition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules
THE JOURNAL OF COMPARATIVE NEUROLOGY 317:1-30 (1992) Five Topographically Organized Fields in the Somatosensory Cortex of the Flying Fox: Microelectrode Maps, Myeloarchitecture, and Cortical Modules LEAH A. KRUBITZER AND MIKE B. CALFORD Vision, Touch and Hearing Research Centre, Department of Physiology and Pharmacology, The University of Queensland, Queensland, Australia 4072 ABSTRACT Five somatosensory fields were defined in the grey-headed flying fox by using microelec- trode mapping procedures. These fields are: the primary somatosensory area, SI or area 3b; a field caudal to area 3b, area 1/2; the second somatosensory area, SII; the parietal ventral area, PV; and the ventral somatosensory area, VS. A large number of closely spaced electrode penetrations recording multiunit activity revealed that each of these fields had a complete somatotopic representation. Microelectrode maps of somatosensory fields were related to architecture in cortex that had been flattened, cut parallel to the cortical surface, and stained for myelin. Receptive field size and some neural properties of individual fields were directly compared. Area 3b was the largest field identified and its topography was similar to that described in many other mammals. Neurons in 3b were highly responsive to cutaneous stimulation of peripheral body parts and had relatively small receptive fields. The myeloarchi- tecture revealed patches of dense myelination surrounded by thin zones of lightly myelinated cortex. Microelectrode recordings showed that myelin-dense and sparse zones in 3b were related to neurons that responded consistently or habituated to repetitive stimulation respectively. In cortex caudal to 3b, and protruding into 3b, a complete representation of the body surface adjacent to much of the caudal boundary of 3b was defined. -

Auditory Experience Controls the Maturation of Song Discrimination and Sexual Response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi*
RESEARCH ARTICLE Auditory experience controls the maturation of song discrimination and sexual response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi* Graduate School of Science, Nagoya University, Nagoya, Japan Abstract In birds and higher mammals, auditory experience during development is critical to discriminate sound patterns in adulthood. However, the neural and molecular nature of this acquired ability remains elusive. In fruit flies, acoustic perception has been thought to be innate. Here we report, surprisingly, that auditory experience of a species-specific courtship song in developing Drosophila shapes adult song perception and resultant sexual behavior. Preferences in the song-response behaviors of both males and females were tuned by social acoustic exposure during development. We examined the molecular and cellular determinants of this social acoustic learning and found that GABA signaling acting on the GABAA receptor Rdl in the pC1 neurons, the integration node for courtship stimuli, regulated auditory tuning and sexual behavior. These findings demonstrate that maturation of auditory perception in flies is unexpectedly plastic and is acquired socially, providing a model to investigate how song learning regulates mating preference in insects. DOI: https://doi.org/10.7554/eLife.34348.001 Introduction Vocal learning in infants or juvenile birds relies heavily on the early experience of the adult conspe- cific sounds. In humans, early language input is necessary to form the ability of phonetic distinction *For correspondence: and pattern detection in the phase of auditory learning (Doupe and Kuhl, 1999; Kuhl, 2004). [email protected] Because of the strong parallels between speech acquisition of humans and song learning of song- Competing interests: The birds, and the difficulties to investigate the neural mechanisms of human early auditory memory at authors declare that no cellular resolution, songbirds have been used as a predominant model in studying memory formation competing interests exist. -
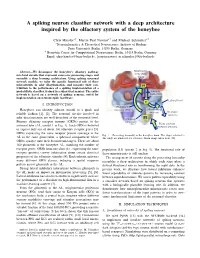
A Spiking Neuron Classifier Network with a Deep Architecture Inspired By
A spiking neuron classifier network with a deep architecture inspired by the olfactory system of the honeybee Chris Hausler¨ ∗†, Martin Paul Nawrot∗† and Michael Schmuker∗† ∗Neuroinformatics & Theoretical Neuroscience, Institute of Biology Freie Universitat¨ Berlin, 14195 Berlin, Germany † Bernstein Center for Computational Neuroscience Berlin, 10115 Berlin, Germany Email: [email protected], fmartin.nawrot, [email protected] Abstract—We decompose the honeybee’s olfactory pathway Mushroom into local circuits that represent successive processing stages and body (MB) calyx resemble a deep learning architecture. Using spiking neuronal network models, we infer the specific functional role of these microcircuits in odor discrimination, and measure their con- 2 tribution to the performance of a spiking implementation of a probabilistic classifier, trained in a supervised manner. The entire Kenyon network is based on a network of spiking neurons, suited for Cells (KC) implementation on neuromorphic hardware. Lateral horn 3 I. INTRODUCTION Projection Honeybees can identify odorant stimuli in a quick and neurons (PN) MB-extrinsic neurons To motor reliable fashion [1], [2]. The neuronal circuits involved in neurons odor discrimination are well described at the structural level. 1 Antennal Primary olfactory receptor neurons (ORNs) project to the lobe (AL) From odorant antennal lobe (AL, circuit 1 in Fig. 1). Each ORN is believed receptor neurons to express only one of about 160 olfactory receptor genes [3]. ORNs expressing the same receptor protein converge in the Fig. 1. Processing hierarchy in the honeybee brain. The stages relevant to AL in the same glomerulus, a spherical compartment where this study are numbered for reference. -

Neural Mapping of Direction and Frequency in the Cricket Cercal Sensory System
The Journal of Neuroscience, March 1, 1999, 19(5):1771–1781 Neural Mapping of Direction and Frequency in the Cricket Cercal Sensory System Sussan Paydar,2 Caitlin A. Doan,2 and Gwen A. Jacobs1 1Center for Computational Biology, Montana State University, Bozeman, Montana 59717, and 2Department of Molecular and Cell Biology, University of California, Berkeley, California 94720 Primary mechanosensory receptors and interneurons in the segregation was investigated, by calculating the relative over- cricket cercal sensory system are sensitive to the direction and lap between the long and medium-length hair afferents with the frequency of air current stimuli. Receptors innervating long dendrites of two interneurons that are known to have different mechanoreceptor hairs (.1000 mm) are most sensitive to low- frequency sensitivities. Both interneurons were shown to have frequency air currents (,150 Hz); receptors innervating nearly equal anatomical overlap with long and medium hair medium-length hairs (900–500 mm) are most sensitive to higher afferents. Thus, the differential overlap of these interneurons frequency ranges (150–400 Hz). Previous studies demonstrated with the two different classes of afferents was not adequate to that the projection pattern of the synaptic arborizations of long explain the observed frequency selectivity of the interneurons. hair receptor afferents form a continuous map of air current Other mechanisms such as selective connectivity between direction within the terminal abdominal ganglion (Jacobs and subsets of afferents and interneurons and/or differences in Theunissen, 1996). We demonstrate here that the projection interneuron biophysical properties must play a role in establish- pattern of the medium-length hair afferents also forms a con- ing the frequency selectivities of these interneurons. -

1P MON. PM Cortex and Subcortical Auditory Nuclei
dynamic role for the vestibular system in orientation and flight control. Laboratory studies of flight behavior under illuminated and dark conditions in both static and rotating obstacle tests were carried out while administering heavy water ͑D2O͒ to bats to impair their vestibular inputs. Eptesicus carried out complex maneuvers through both fixed arrays of wires and a rotating obstacle array using both vision and echolocation, or when guided by echolocation alone. When treated with D2O in combination with lack of visual cues, bats showed considerable decrements in performance. These data indicate that big brown bats use both vision and echolocation to provide spatial registration for head position information generated by the vestibular system. 2:55 1pAB3. Bat’s auditory system: Corticofugal feedback and plasticity. Nobuo Suga ͑Dept. of Biol., Washington Univ., One Brookings Dr., St. Louis, MO 63130͒ The auditory system of the mustached bat consists of physiologically distinct subdivisions for processing different types of biosonar information. It was found that the corticofugal ͑descending͒ auditory system plays an important role in improving and adjusting auditory signal processing. Repetitive acoustic stimulation, cortical electrical stimulation or auditory fear conditioning evokes plastic changes of the central auditory system. The changes are based upon egocentric selection evoked by focused positive feedback associated with lateral inhibition. Focal electric stimulation of the auditory cortex evokes short-term changes in the auditory 1p MON. PM cortex and subcortical auditory nuclei. An increase in a cortical acetylcholine level during the electric stimulation changes the cortical changes from short-term to long-term. There are two types of plastic changes ͑reorganizations͒: centripetal best frequency shifts for expanded reorganization of a neural frequency map and centrifugal best frequency shifts for compressed reorganization of the map. -

Portia Perceptions: the Umwelt of an Araneophagic Jumping Spider
Portia Perceptions: The Umwelt of an Araneophagic Jumping 1 Spider Duane P. Harland and Robert R. Jackson The Personality of Portia Spiders are traditionally portrayed as simple, instinct-driven animals (Savory, 1928; Drees, 1952; Bristowe, 1958). Small brain size is perhaps the most compelling reason for expecting so little flexibility from our eight-legged neighbors. Fitting comfortably on the head of a pin, a spider brain seems to vanish into insignificance. Common sense tells us that compared with large-brained mammals, spiders have so little to work with that they must be restricted to a circumscribed set of rigid behaviors, flexibility being a luxury afforded only to those with much larger central nervous systems. In this chapter we review recent findings on an unusual group of spiders that seem to be arachnid enigmas. In a number of ways the behavior of the araneophagic jumping spiders is more comparable to that of birds and mammals than conventional wisdom would lead us to expect of an arthropod. The term araneophagic refers to these spiders’ preference for other spiders as prey, and jumping spider is the common English name for members of the family Saltici- dae. Although both their common and the scientific Latin names acknowledge their jumping behavior, it is really their unique, complex eyes that set this family of spiders apart from all others. Among spiders (many of which have very poor vision), salticids have eyes that are by far the most specialized for resolving fine spatial detail. We focus here on the most extensively studied genus, Portia. Before we discuss the interrelationship between the salticids’ uniquely acute vision, their predatory strategies, and their apparent cognitive abilities, we need to offer some sense of what kind of animal a jumping spider is; to do this, we attempt to offer some insight into what we might call Portia’s personality. -

Krogh's Principle
Introduction to Neuroscience: Behavioral Neuroscience Neuroethology, Comparative Neuroscience, Natural Neuroscience Nachum Ulanovsky Department of Neurobiology, Weizmann Institute of Science 2017-2018, 2nd semester Principles of Neuroethology Neuroethology seeks to understand the mechanisms by which the Neurobiology central nervous system controls the Neuroethology Ethology natural behavior of animals. • Focus on Natural behaviors: Choosing to study a well-defined and reproducible yet natural behavior (either Innate or Learned behavior) • Need to study thoroughly the animal’s behavior, including in the field: Neuroethology starts with a good understanding of Ethology. • If you study the animals in the lab, you need to keep them in conditions as natural as possible, to avoid the occurrence of unnatural behaviors. • Krogh’s principle 1 Krogh’s principle August Krogh Nobel prize 1920 “For such a large number of problems there will be some animal of choice or a few such animals on which it can be most conveniently studied. Many years ago when my teacher, Christian Bohr, was interested in the respiratory mechanism of the lung and devised the method of studying the exchange through each lung separately, he found that a certain kind of tortoise possessed a trachea dividing into the main bronchi high up in the neck, and we used to say as a laboratory joke that this animal had been created expressly for the purposes of respiration physiology. I have no doubt that there is quite a number of animals which are similarly "created" for special physiological -

Lateral Inhibition Effects Demonstrate That the Maturational State of Neurons That Then Inhibit Their Neighbors
522 Lateral Inhibition effects demonstrate that the maturational state of neurons that then inhibit their neighbors. When a the learner’s brain is crucial for the attainment of stimulus (such as a bar of light or any other stim- a language system. ulus) excites a number of neurons in the network Several questions remain open about the mecha- (in this case neurons 4e, 5e, 6e, and 7e), the effect nisms underlying language learning. One issue is of inhibition is to suppress the neurons just out- whether specific aspects of language acquisition side the edge of the bar (3e and 8e) because those should be attributed to language-specific versus neurons are inhibited but not excited. Further, general-purpose learning mechanisms. Another issue because the neurons just inside the edges of the is whether children’s native language can affect the bar (4e and 7e) are excited by light and only inhib- way they think, and whether language is necessary ited by one neighbor, they are especially active. or helpful for the development of human concepts. This leads to perceptual contrast enhancement at borders. Further research showed that lateral inhi- Anna Papafragou bition also applied to overlapping stimuli, and that its strength fell off with distance between the See also Aphasias; Audition: Cognitive Influences; Context Effects in Perception; Speech Perception; Top- interacting stimuli. Down and Bottom-Up Processing; Word Recognition Haldan Keffer Hartline won the Nobel Prize in 1967 for discovering lateral inhibition and its neu- ral correlates. The first inhibitory circuit in the Further Readings nervous system was found in the horseshoe crab (Limulus polyphemus). -

Sensory Receptors A17 (1)
SENSORY RECEPTORS A17 (1) Sensory Receptors Last updated: April 20, 2019 Sensory receptors - transducers that convert various forms of energy in environment into action potentials in neurons. sensory receptors may be: a) neurons (distal tip of peripheral axon of sensory neuron) – e.g. in skin receptors. b) specialized cells (that release neurotransmitter and generate action potentials in neurons) – e.g. in complex sense organs (vision, hearing, equilibrium, taste). sensory receptor is often associated with nonneural cells that surround it, forming SENSE ORGAN. to stimulate receptor, stimulus must first pass through intervening tissues (stimulus accession). each receptor is adapted to respond to one particular form of energy at much lower threshold than other receptors respond to this form of energy. adequate (s. appropriate) stimulus - form of energy to which receptor is most sensitive; receptors also can respond to other energy forms, but at much higher thresholds (e.g. adequate stimulus for eye is light; eyeball rubbing will stimulate rods and cones to produce light sensation, but threshold is much higher than in skin pressure receptors). when information about stimulus reaches CNS, it produces: a) reflex response b) conscious sensation c) behavior alteration SENSORY MODALITIES Sensory Modality Receptor Sense Organ CONSCIOUS SENSATIONS Vision Rods & cones Eye Hearing Hair cells Ear (organ of Corti) Smell Olfactory neurons Olfactory mucous membrane Taste Taste receptor cells Taste bud Rotational acceleration Hair cells Ear (semicircular -

Early Events in Olfactory Processing
ANRV278-NE29-06 ARI 8 May 2006 15:38 Early Events in Olfactory Processing Rachel I. Wilson1 and Zachary F. Mainen2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115; email: rachel [email protected] 2Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724; email: [email protected] Annu. Rev. Neurosci. Key Words 2006. 29:163–201 olfactory bulb, antennal lobe, chemotopy, temporal coding, The Annual Review of Neuroscience is online at synchrony, concentration, segmentation by HARVARD UNIVERSITY on 07/17/06. For personal use only. neuro.annualreviews.org Abstract doi: 10.1146/ annurev.neuro.29.051605.112950 Olfactory space has a higher dimensionality than does any other class Annu. Rev. Neurosci. 2006.29:163-201. Downloaded from arjournals.annualreviews.org Copyright c 2006 by of sensory stimuli, and the olfactory system receives input from an Annual Reviews. All rights unusually large number of unique information channels. This sug- reserved gests that aspects of olfactory processing may differ fundamentally 0147-006X/06/0721- from processing in other sensory modalities. This review summa- 0163$20.00 rizes current understanding of early events in olfactory processing. We focus on how odors are encoded by the activity of primary olfac- tory receptor neurons, how odor codes may be transformed in the olfactory bulb, and what relevance these codes may have for down- stream neurons in higher brain centers. Recent findings in synaptic physiology, neural coding, and psychophysics are discussed, with ref- erence to both vertebrate and insect model systems. 163 ANRV278-NE29-06 ARI 8 May 2006 15:38 Contents CHALLENGES TO Systematic Progression in UNDERSTANDING Molecular Feature OLFACTORY PROCESSING . -

The Retina the Retina the Pigment Layer Contains Melanin That Prevents Light Reflection Throughout the Globe of the Eye
The Retina The retina The pigment layer contains melanin that prevents light reflection throughout the globe of the eye. It is also a store of Vitamin A. Rods and Cones Photoreceptors of the nervous system responsible for transforming light energy into the electrical energy of the nervous system. Bipolar cells Link between photoreceptors and ganglion cells. Horizontal cells Provide inhibition between bipolar cells. This is the mechanism of lateral inhibition which is important to edge detection and contrast enhancement. Amacrine cells Transmit excitatory signals from bipolar to ganglion cells. Thought to be important in signalling changes in light intensity. Neural circuitry About 125 rods and 5 cones converge on each optic nerve fibre. In the central portion of the fovea there are no rods. The ratio of cones to optic nerves in the fovea is one. This increases the visual acuity of the fovea. Rods and Cones Photosensitive substance in rods is called rhodopsin and in cones is called iodopsin. The Photochemistry of Vision Rhodopsin and Iodopsin Cis-retinal +Opsin Light sensitive Light Trans-retinal Opsin Light insensitive This is a reversible reaction Cis-retinal +Opsin Light sensitive Vitamin A In the presence of light, the rods and cones are hyperpolarized mv t Hyperpolarizing potential lasts for upto half a second, leading to the perception of fusion of flickering lights. Receptor potential – rod and cone potential Hyperpolarizing receptor potential caused by rhodopsin decomposition. Hyperpolarizing potential lasts for upto half a second, leading to the fusion of flickering lights. Light and dark adaptation in the visual system The eye is capable of vision in conditions of light that vary greatly in intensity The eye adapts dynamically to lighting conditions Light and Dark Adaptation We are able to see in light intensities that vary greatly. -

Small and Dim Target Detection Via Lateral Inhibition Filtering and Artificial Bee Colony Based Selective Visual Attention
Small and Dim Target Detection via Lateral Inhibition Filtering and Artificial Bee Colony Based Selective Visual Attention Haibin Duan1,2*, Yimin Deng1,2, Xiaohua Wang2, Chunfang Xu1 1 State Key Laboratory of Virtual Reality Technology and Systems, School of Automation Science and Electrical Engineering, Beihang University (formerly Beijing University of Aeronautics and Astronautics), Beijing, P. R. China, 2 Science and Technology on Aircraft Control Laboratory, Beihang University, Beijing, P. R. China Abstract This paper proposed a novel bionic selective visual attention mechanism to quickly select regions that contain salient objects to reduce calculations. Firstly, lateral inhibition filtering, inspired by the limulus’ ommateum, is applied to filter low- frequency noises. After the filtering operation, we use Artificial Bee Colony (ABC) algorithm based selective visual attention mechanism to obtain the interested object to carry through the following recognition operation. In order to eliminate the camera motion influence, this paper adopted ABC algorithm, a new optimization method inspired by swarm intelligence, to calculate the motion salience map to integrate with conventional visual attention. To prove the feasibility and effectiveness of our method, several experiments were conducted. First the filtering results of lateral inhibition filter were shown to illustrate its noise reducing effect, then we applied the ABC algorithm to obtain the motion features of the image sequence. The ABC algorithm is proved to be more robust and effective through the comparison between ABC algorithm and popular Particle Swarm Optimization (PSO) algorithm. Except for the above results, we also compared the classic visual attention mechanism and our ABC algorithm based visual attention mechanism, and the experimental results of which further verified the effectiveness of our method.