Effect of the Expiratory Positive Airway Pressure on Dynamic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

People, Plagues, and Prices in the Roman World: the Evidence from Egypt
People, Plagues, and Prices in the Roman World: The Evidence from Egypt KYLE HARPER The papyri of Roman Egypt provide some of the most important quantifiable data from a first-millennium economy. This paper builds a new dataset of wheat prices, land prices, rents, and wages over the entire period of Roman control in Egypt. Movements in both nominal and real prices over these centuries suggest periods of intensive and extensive economic growth as well as contraction. Across a timeframe that covers several severe mortality shocks, demographic changes appear to be an important, but by no means the only, force behind changes in factor prices. his article creates and analyzes a time series of wheat and factor Tprices for Egypt from AD 1 to the Muslim conquest, ~AD 641. From the time the territory was annexed by Octavian in 30 BCE until it was permanently taken around AD 641, Egypt was an important part of the Roman Empire. Famously, it supplied grain for the populations of Rome and later Constantinople, but more broadly it was integrated into the culture, society, and economy of the Roman Mediterranean. While every province of the sprawling Roman Empire was distinctive, recent work stresses that Egypt was not peculiar (Bagnall 1993; Rathbone 2007). Neither its Pharaonic legacy, nor the geography of the Nile valley, make it unrepresentative of the Roman world. In one crucial sense, however, Roman Egypt is truly unique: the rich- ness of its surviving documentation. Because of the valley’s arid climate, tens of thousands of papyri, covering the entire spectrum of public and private documents, survive from the Roman period (Bagnall 2009). -

Varipos 250S+270S 20191115
Smart Dual Hinged design for the ultimate flexibility 250 S / 270S Features Flexibility meets demand with Hidden Cable Management - dual hinged stand allowing extreme tilt Keeping Everything Organized Easy adjustment providing a solid platform allowing extremely The Dual Hinge Stand provides hidden cable management flexible height and angle adjustment. The stand can be folded flat channels to organize & reduce clutter when connecting or adjusted to any view angle providing maximum comfort and power & accessory cables; the cables are concealed within optimal efficiency to the POS Operation. the stand and covered with a securing plate maintaining the aesthetic look. Powerful and Flexible VariIO I/O Box Connectivity - The Ideal Simplified Installation and Serviceability Retail-Ready Solution Simplified Installation and Serviceability is designed into the VariPOS 250S / 270S providing quick and easy service for routine VariPOS250S / 270S can be combined with the dedicated VariIO I/O maintenance schedules significantly reducing engineer onsite visit Box offering the standard I/O connections plus Point of Sale specific time. VariIO I/O Box connected by a single custom high speed connections for Powered POS peripherals. The Extendable I/O USB Type C connector can easily be disconnected leaving the I/O capability meets multiple project specification with simple onboard and peripherals in place panel I/O, advanced VariIO I/O Box functionality & basic I/O box compatibility. Detailed features Ultra-flexible Dual-hinge Design for Compact & Stylish Systems Intel 6th Generation Celeron i3, i5 high-end processors in a fanless structure True flat 15” 4:3 LCD, 400 nits / 17’’ 5:4 LCD, 400 nits (LED backlight) with IP66 protection. -

Measuring the Power of the Roman Empire
26 Potter Chapter 2 Measuring the Power of the Roman Empire David Potter By the age of Cicero – the point at which they began to recognize the fact that they controlled a territorial empire and could raise revenue from it – the Ro- mans were accustomed to measure their power with criteria taken over whole- sale from Greek theory. In the De Legibus, Cicero says simply that senators ought to know the state of the army, the treasury, the allies, friends and tribu- taries of Rome and the nature of the attachment of each to Rome. For Cicero this is what it was to “know the State.”1 In these terms power is a function of income and manpower. Such a measurement, which, as we shall see, would have a long history going forward, was even then being amply employed by Gaius Caesar in his Gallic Wars, and had a long prior history in Greek thought. Caesar famously illustrated the power of the various Gallic and German tribes he subdued or encountered by telling his readers how many of them there were or had been. Good of the Helvetians to have produced a census docu- ment (in Greek no less) attesting to the fact that there were 368,000 of them at the beginning of their migration. There were now a mere 110,000 going home. The Suebi, the most powerful of the Germans were said (a nice concession on Caesar’s part) to have controlled one hundred districts which each furnished 1000 men to fight each year – since the same warriors only fought every other year, this meant that there were at least 200,000 of them, and there were nearly 250,000 Gauls who came to the relief of Vercingetorix (including men from tribes such as the Nervii that Caesar claimed to have annihilated in recent years).2 A conception of state power as a function of demography and money would have been familiar to any Roman aristocrat who knew (as any Roman aristo- crat would have) the works of fifth-century Greek historians. -

Presidential Address 2014 Coin Hoards and Hoarding in Britain
PRESIDENTIAL ADDRESS 2014 COIN HOARDS AND HOARDING IN BRITAIN (3): RADIATE HOARDS ROGER BLAND Introduction IN my first presidential address I gave an overview of hoarding in Britain from the Bronze Age through to recent times,1 while last year I spoke about hoards from the end of Roman Britain.2 This arises from a research project (‘Crisis or continuity? Hoards and hoarding in Iron Age and Roman Britain’) funded by the Arts and Humanities Research Council and based at the British Museum and the University of Leicester. The project now includes the whole of the Iron Age and Roman periods from around 120 BC to the early fifth century. For the Iron Age we have relied on de Jersey’s corpus of 340 Iron Age coin hoards and we are grateful to him for giving us access to his data in advance of publica- tion.3 For the Roman period, our starting point has been Anne Robertson’s Inventory of Romano-British Coin Hoards (RBCH),4 which includes details of 2,007 hoards, including dis- coveries made down to about 1990. To that Eleanor Ghey has added new discoveries and also trawled other data sources such as Guest and Wells’s corpus of Roman coin finds from Wales,5 David Shotter’s catalogues of Roman coin finds from the North West,6 Penhallurick’s corpus of Cornish coin finds,7 Historic Environment Records and the National Monuments Record. She has added a further 1,045 Roman hoards, taking the total to 3,052, but this is not the final total. -

List of Approved Fire Alarm Companies
Approved Companies List Fire Alarm Company Wednesday, September 1, 2021 ____________________________________________________ App No. 198S Approval Exp: 2/22/2022 Company : AAA FIRE & SECURITY SYSTEMS Address: 67 WEST STREET UNIT 321 Brooklyn, NY 11222 Telephone #: 718-349-5950 Principal's Name: NAPHTALI LICHTENSTEIN “S” after the App No. means that Insurance Exp Date: 6/21/2022 the company is also an FDNY approved smoke detector ____________________________________________________ maintenance company. App No. 268S Approval Exp: 4/16/2022 Company : ABLE FIRE PREVENTION CORP. Address: 241 WEST 26TH STREET New York, NY 10001 Telephone #: 212-675-7777 Principal's Name: BRIAN EDWARDS Insurance Exp Date: 9/22/2021 ____________________________________________________ App No. 218S Approval Exp: 3/3/2022 Company : ABWAY SECURITY SYSTEM Address: 301 MCLEAN AVENUE Yonkers, NY 10705 Telephone #: 914-968-3880 Principal's Name: MARK STERNEFELD Insurance Exp Date: 2/3/2022 ____________________________________________________ App No. 267S Approval Exp: 4/14/2022 Company : ACE ELECTRICAL CONSTRUCTION Address: 130-17 23RD AVENUE College Point, NY 11356 Telephone #: 347-368-4038 Principal's Name: JEFFREY SOCOL Insurance Exp Date: 12/14/2021 30 days within today’s date Page 1 of 34 ____________________________________________________ App No. 243S Approval Exp: 3/23/2022 Company : ACTIVATED SYSTEMS INC Address: 1040 HEMPSTEAD TPKE, STE LL2 Franklin Square, NY 11010 Telephone #: 516-538-5419 Principal's Name: EDWARD BLUMENSTETTER III Insurance Exp Date: 5/12/2022 ____________________________________________________ App No. 275S Approval Exp: 4/27/2022 Company : ADR ELECTRONICS LLC Address: 172 WEST 77TH STREET #2D New York, NY 10024 Telephone #: 212-960-8360 Principal's Name: ALAN RUDNICK Insurance Exp Date: 1/31/2022 ____________________________________________________ App No. -

Harttimo 1.Pdf
Beyond the River, under the Eye of Rome Ethnographic Landscapes, Imperial Frontiers, and the Shaping of a Danubian Borderland by Timothy Campbell Hart A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Greek and Roman History) in the University of Michigan 2017 Doctoral Committee: Professor David S. Potter, Co-Chair Professor Emeritus Raymond H. Van Dam, Co-Chair Assistant Professor Ian David Fielding Professor Christopher John Ratté © Timothy Campbell Hart [email protected] ORCID iD: 0000-0002-8640-131X For my family ii ACKNOWLEDGEMENTS Developing and writing a dissertation can, at times, seem like a solo battle, but in my case, at least, this was far from the truth. I could not have completed this project without the advice and support of many individuals, most crucially, my dissertation co-chairs David S. Potter, and Raymond Van Dam. Ray saw some glimmer of potential in me and worked to foster it from the moment I arrived at Michigan. I am truly thankful for his support throughout the years and constant advice on both academic and institutional matters. In particular, our conversations about demographics and the movement of people in the ancient world were crucial to the genesis of this project. Throughout the writing process, Ray’s firm encouragement towards clarity of argument and style, while not always what I wanted to hear, have done much to make this a stronger dissertation. David Potter has provided me with a lofty academic model towards which to strive. I admire the breadth and depth of his scholarship; working and teaching with him have shown me much worth emulating. -

A COMPANION to the ROMAN ARMY Edited By
ACTA01 8/12/06 11:10 AM Page iii A COMPANION TO THE ROMAN ARMY Edited by Paul Erdkamp ACTA01 8/12/06 11:10 AM Page i A COMPANION TO THE ROMAN ARMY ACTA01 8/12/06 11:10 AM Page ii BLACKWELL COMPANIONS TO THE ANCIENT WORLD This series provides sophisticated and authoritative overviews of periods of ancient history, genres of classical lit- erature, and the most important themes in ancient culture. Each volume comprises between twenty-five and forty concise essays written by individual scholars within their area of specialization. The essays are written in a clear, provocative, and lively manner, designed for an international audience of scholars, students, and general readers. Ancient History Published A Companion to the Roman Army A Companion to the Classical Greek World Edited by Paul Erdkamp Edited by Konrad H. Kinzl A Companion to the Roman Republic A Companion to the Ancient Near East Edited by Nathan Rosenstein and Edited by Daniel C. Snell Robert Morstein-Marx A Companion to the Hellenistic World A Companion to the Roman Empire Edited by Andrew Erskine Edited by David S. Potter In preparation A Companion to Ancient History A Companion to Late Antiquity Edited by Andrew Erskine Edited by Philip Rousseau A Companion to Archaic Greece A Companion to Byzantium Edited by Kurt A. Raaflaub and Hans van Wees Edited by Elizabeth James A Companion to Julius Caesar Edited by Miriam Griffin Literature and Culture Published A Companion to Catullus A Companion to Greek Rhetoric Edited by Marilyn B. Skinner Edited by Ian Worthington A Companion to Greek Religion A Companion to Ancient Epic Edited by Daniel Ogden Edited by John Miles Foley A Companion to Classical Tradition A Companion to Greek Tragedy Edited by Craig W. -

The Ruin of the Roman Empire
7888888888889 u o u o u o u THE o u Ruin o u OF THE o u Roman o u o u EMPIRE o u o u o u o u jamesj . o’donnell o u o u o u o u o u o u o hjjjjjjjjjjjk This is Ann’s book contents Preface iv Overture 1 part i s theoderic’s world 1. Rome in 500: Looking Backward 47 2. The World That Might Have Been 107 part ii s justinian’s world 3. Being Justinian 177 4. Opportunities Lost 229 5. Wars Worse Than Civil 247 part iii s gregory’s world 6. Learning to Live Again 303 7. Constantinople Deflated: The Debris of Empire 342 8. The Last Consul 364 Epilogue 385 List of Roman Emperors 395 Notes 397 Further Reading 409 Credits and Permissions 411 Index 413 About the Author Other Books by James J. O’ Donnell Credits Cover Copyright About the Publisher preface An American soldier posted in Anbar province during the twilight war over the remains of Saddam’s Mesopotamian kingdom might have been surprised to learn he was defending the westernmost frontiers of the an- cient Persian empire against raiders, smugglers, and worse coming from the eastern reaches of the ancient Roman empire. This painful recycling of history should make him—and us—want to know what unhealable wound, what recurrent pathology, what cause too deep for journalists and politicians to discern draws men and women to their deaths again and again in such a place. The history of Rome, as has often been true in the past, has much to teach us. -
Surgical Technique Guide
Surgical Technique 1 THE MARKET LEADER WORLDWIDE FOR TRAUMA2 Table of Contents Introduction Radial Head Replacement System 2 Indications 4 Operative Planning 5 Surgical Technique Surgical Technique 8 Optional Removal of Planer From Sounder 20 Implant Removal 21 Potential Adverse Events 22 Product Information Implants 23 Instrument Kit 24 Sets 24 Carrying Case 25 Additional Sets Available 25 MR Information The Radial Head Replacement System has not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration or image artifact in the MR environment. The safety of the Radial Head Replacement System in the MR environment is unknown. Scanning a patient who has this device may result in patient injury. Image intensifier control Radial Head Replacement System Surgical Technique DePuy Synthes 1 Radial Head Replacement System The DePuy Synthes Radial Head Replacement System is a solution for replacing the Radial Head in patients. The treatment goals for radial head fractures are to restore elbow stability and forearm rotation, to preserve elbow motion, and to maintain the length of the radius. The DePuy Synthes Radial Head Replacement System is used to treat patients who have experienced destabilizing radial head fractures that cannot be repaired. The Radial Head Replacement is also useful in the elective management of elbow arthritis in the radio-capitellar joint. Our solution is designed to support against longitudinal collapse of the radius allowing associated soft tissue injuries to heal with the radial head in an anatomic position.3 There are two main implant philosophies for radial head replacements. Rigid fixation with a straight or anatomically curved stem that can be press-fit or cemented, and loose fitting with a straight smooth stem. -

Budget Detail
CITY OF PHILADELPHIA ORGANIZATION CHART (ALL FUNDS) BY PROGRAM FISCAL 2022 OPERATING BUDGET Department No. Office of the Director of Finance 35 Director of Finance 121 133 Budget Office Accounting Bureau Executive Finance Project Risk Management 17 19 Direction Management Office Analysis & 39 39 Communication 39 49 6 6 20 20 Financial SECTION 15 Verification Operating Budget Payroll Bureau Claims General Accounting Capital Budget OnePhilly Employee Unit Disability Grants Programs Accounting & Office of Recovery Administration and Grants Risk Management & Insurance Administrative Service Center Safety & Loss Prevention Internal Controls & Compliance FY 22 BUDGET ORGANIZATION FY21 FY22 FILLED BUDGETED POS. 12/20 POSITIONS 121 133 71-53A 1 CITY OF PHILADELPHIA DEPARTMENTAL SUMMARY BY FUND FISCAL 2022 OPERATING BUDGET Department No. Office of the Director of Finance 35 Fiscal 2020 Fiscal 2021 Fiscal 2021 Fiscal 2022 Increase Actual Original Estimated Proposed or No. Fund Class Description Obligations Appropriation Obligations Budget (Decrease) (1) (2) (3) (4) (5) (6) (7) (8) (9) 01 100 Employee Compensation a) Personal Services 10,314,652 12,962,396 11,567,135 12,519,056 951,921 GENERAL b) Employee Benefits 1,363,379,633 1,287,159,003 1,302,161,016 1,434,553,835 132,392,819 200 Purchase of Services 3,138,973 2,798,178 3,193,439 3,610,785 417,346 300 Materials and Supplies 40,489 30,000 30,000 30,000 400 Equipment 5,752 5,616 5,616 5,616 500 Contributions, etc. 276,106,724 347,459,717 362,705,074 355,017,976 (7,687,098) 800 Payments to Other Funds 115,561,000 24,500,000 24,500,000 11,360,000 (13,140,000) 900 Advances & Other Misc 25,000,000 75,000,000 75,000,000 Total 1,768,547,223 1,699,914,910 1,704,162,280 1,892,097,268 187,934,988 02 100 Employee Compensation a) Personal Services b) Employee Benefits 145,059,709 135,546,760 144,111,226 126,977,257 (17,133,969) WATER 200 Purchase of Services 300 Materials and Supplies 400 Equipment 500 Contributions, etc. -
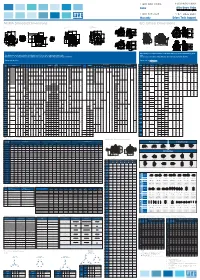
NEMA Standard Dimensions IEC Critical Dimensions
1-800-ASK-4WEG 1-800-ASK-4WEG Sales After Hours Sales Select after hours option 1-800-839-2529 1-877-WEG-DRIV Warranty Drives Tech Support NEMA Standard Dimensions IEC Critical Dimensions Frames 143T to 444/5T Frames 445/7T to 586/7T Frame 588/9T C Flange B5 Flange (FF) WEG W22 Terminal Box WEG W22 Terminal Box Reference Reference D Flange B14 Flange (C-DIN) This drawing is not representative of all frame sizes. It is used as a dimension guide This drawing is not representative of all frame sizes. It is used as a dimension guide only. only. Dimension C, O, P and all Terminal Box dimensions are specific to WEG W22 General Purpose Motors. Dimension L is specific to WEG W22 IEC General Purpose Metric Motors. Dimensions in inches WARRANTY Dimensions in millimeters inches SHAFT “C” FLANGE DIMENSIONS “D” FLANGE DIMENSIONS Shaft B5 Flange (FF) Dimensions B14 Flange (C-DIN) Dimensions NEMA MOUNTING KEYWAY TERMINAL BOX BEARINGS Frame A AC B C L ABCDGJOKPT EXTENSION d1 BF BF DE Flange M N P Flange M N P Frames BA AJ AK BD BB BC AH BA AJ AK BD BB 100 125 80 40 11 23 216 115 95 140 75 60 90 2E 2F H BA S R ES N-W U AB HB HF HG HH HK LL LM AA D.E. N.D.E NUMBER TAP SIZE NUMBER TAP SIZE FF-115 C-90 63 3.937 4.921 3.150 0.433 0.433 0.906 8.504 4.528 3.740 5.512 2.953 2.362 3.543 143T 4.000 5.157 12.346 4.250 2.250(A) UNC 112 141 90 45 14 30 248 130 110 160 85 70 105 5.500 0.344 2.250 6.457 3.500 0.354 1.437 7.122 7.047 - 0.187 0.765 1.575 2.250 0.875 6.181 1.728 3.500 2.638 4.527 4.094 NPT3/4" 6205 ZZ 6204 ZZ 5.875 4.500 6.500 0.156 2.125 2.250 71 FF-130 -

Summer 2021: Gen Ed Courses This List Is Subject to Change
Summer 2021: Gen Ed Courses This list is subject to change. Please check Course Search to confirm Subject Title CRN Course Section Credits Attribute Start Date End Date College Instructor African-American Studies-AAST Black: Africa to Hip-Hop 51068 141H 50D 3 Historical Studies (H) May 10, 2021 June 18, 2021 College Humanities & Sciences Shearer, Tobin African-American Studies-AAST Black: Africa to Hip-Hop 51068 141H 50D 3 Cultural Intl Diversity (X) May 10, 2021 June 18, 2021 College Humanities & Sciences Shearer, Tobin Allied Health Med Support-AHMS Medical Ethics 50432 270E 50B 3 Ethical & Human Values Course May 10, 2021 June 18, 2021 Missoula College Hanson, Mark Anthropology (ANTY) Anthro & the Human Experience 51166 101H 01B 3 Historical Studies (H) May 10, 2021 June 18, 2021 College Humanities & Sciences Kerr, David Anthropology (ANTY) Anthro & the Human Experience 51166 101H 01B 3 Cultural Intl Diversity (X) May 10, 2021 June 18, 2021 College Humanities & Sciences Kerr, David Anthropology (ANTY) Anthro & the Human Experience 50911 101H 50E 3 Cultural Intl Diversity (X) June 21, 2021 July 30, 2021 College Humanities & Sciences Laue, Cheyenne Anthropology (ANTY) Anthro & the Human Experience 50911 101H 50E 3 Historical Studies (H) June 21, 2021 July 30, 2021 College Humanities & Sciences Laue, Cheyenne Anthropology (ANTY) Race and Minorities 50565 122S 01B 3 Democracy and Citizenship (Y) May 10, 2021 June 18, 2021 College Humanities & Sciences Campbell, Gregory Anthropology (ANTY) Race and Minorities 50565 122S 01B 3 Social Sciences