Information on NADCP for FMD and Brucellosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
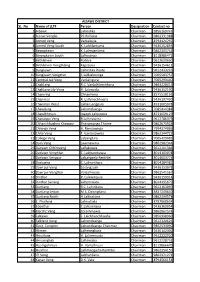
SL. No Name of LLTF Person Designation Contact No 1 Aibawk
AIZAWL DISTRICT SL. No Name of LLTF Person Designation Contact no 1 Aibawk Lalrindika Chairman 9856169747 2 Aizawl Venglai PC Ralliana Chairman 9862331988 3 Armed Veng Vanlalbula Chairman 8794424292 4 Armed Veng South K. Lalthlantuma Chairman 9436152893 5 Bawngkawn K. Lalmuankima Chairman 9862305744 6 Bawngkawn South Lalrosanga Chairman 8118986473 7 Bethlehem Rohlira Chairman 9612629630 8 Bethlehem Vengthlang Kapzauva Chairman 9436154611 9 Bungkawn Lalrindika Royte Chairman 9612433243 10 Bungkawn Vengthar C.Lalbiaknunga Chairman 7005583757 11 Centtral Jail Veng Vanlalngura Chairman 7005293440 12 Chaltlang R.C. Vanlalhlimchhana Chairman 9863228015 13 Chaltlang Lily Veng H. Lalenvela Chairman 9436152190 14 Chamring Chhanhima Chairman 8575518166 15 Chanmari R. Lalhmachhuana Chairman 9436197490 16 Chanmari West Lalliansangpuia Chairman 8731005978 17 Chawilung Lalnuntluanga Chairman 7085414388 18 Chawlhhmun Joseph Lalnunzira Chairman 8731059129 19 Chawnpui Veng R.Lalrinawma Chairman 9612786379 20 Chhanchhuahna Khawpui Thangmanga Thome Chairman 9862673924 21 Chhinga Veng H. Ramzawnga Chairman 7994374886 22 Chite Veng F. Vanlalsawma Chairman 9862344723 23 College Veng Lalsanglura Chairman 7005429082 24 Dam Veng Lawmawma Chairman 9862982344 25 Darlawn Chhimveng Lalfakzuala Chairman 9612201386 26 Darlawn Venghlun C. Lalchanmawia Chairman 8014103078 27 Darlawn Vengpui Lalsangzela Renthlei Chairman 8014603774 28 Darlawng C. Lalnunthara Chairman 8014184382 29 Dawrpui Veng Zosangzuali Chairman 9436153078 30 Dawrpui Vengthar Vanlalhruaia Chairman 9862541567 31 Dinthar R. Lalawmpuia Chairman 9436159914 32 Dinthar Sairang Lalremruata Chairman 8014195679 33 Durtlang R.C. Lalrinliana Chairman 9612163099 34 Durtlang Leitan M.S. Dawngliana Chairman 8837209640 35 Durtlang North H.Lalthakima Chairman 9862399578 36 E. Phaileng Lalruatzela Chairman 8787868634 37 Edenthar C.Lalramliana Chairman 9436360954 38 Electric Veng Zorammawia Chairman 9862867574 39 Falkawn F. Lalchhanchhuaha Chairman 9856998960 40 Falkland Veng Lalnuntluanga Chairman 9612320626 41 Govt. -

AIZAWL DISTRICT No
Allocation of Seats for General Election to Village Councils, 2015 AIZAWL DISTRICT No. of Village Councils: 95 Name of No. of Seats No. of Voters No of District S/N No. & Name of Village Councils Total Household General Reserved Male Female Total with code Seats 1 MZ-VC 01/1 - Aibawk 271 4 1 5 498 489 987 2 MZ-VC 01/2 - Buhban 105 2 1 3 211 220 431 3 MZ-VC 01/3 - Chamring 44 2 1 3 94 99 193 4 MZ-VC 01/4 - Chawilung 85 2 1 3 165 130 295 5 MZ-VC 01/5 - Chhanchhuahna Khawpui 33 2 1 3 56 44 100 6 MZ-VC 01/6 - Daido 79 2 1 3 196 182 378 7 MZ-VC 01/7 - Damdiai (Vervek) 46 2 1 3 84 85 169 8 MZ-VC 01/8 - Darlawn ChhimVeng 104 2 1 3 201 207 408 9 MZ-VC 01/9 - Darlawn Venghlun 314 4 1 5 614 649 1263 10 MZ-VC 01/10 - Darlawn Vengpui 338 4 1 5 593 648 1241 11 MZ-VC 01/11 - Darlawng 132 2 1 3 179 207 386 12 MZ-VC 01/12 - Dilkhan 37 2 1 3 84 74 158 13 MZ-VC 01/13 - E. Phaileng 213 4 1 5 431 444 875 14 MZ-VC 01/14 - Falkawn 260 4 1 5 399 433 832 15 MZ-VC 01/15 - Hmuifang 47 2 1 3 96 104 200 16 MZ-VC 01/16 - Hmunnghak 58 2 1 3 120 122 242 17 MZ-VC 01/17 - Hualngohmun 152 2 1 3 264 291 555 18 MZ-VC 01/18 - Keifang 717 5 2 7 1199 1358 2557 19 MZ-VC 01/19 - Kelsih 141 2 1 3 250 260 510 20 MZ-VC 01/20 - Kepran 150 2 1 3 273 289 562 21 MZ-VC 01/21 - Khanpui 260 4 1 5 524 505 1029 MZ-VC 01 - AIZAWL 22 MZ-VC 01/22 - Khawlian 292 4 1 5 668 592 1260 23 MZ-VC 01/23 - Khawpuar 76 2 1 3 150 147 297 24 MZ-VC 01/24 - Khawruhlian 518 5 2 7 786 794 1580 25 MZ-VC 01/25 - Lailak 96 2 1 3 163 144 307 26 MZ-VC 01/26 - Lamchhip 125 2 1 3 282 251 533 27 MZ-VC 01/27 - Lamherh 106 2 1 3 191 153 344 28 MZ-VC 01/28 - Lenchim 65 2 1 3 109 99 208 29 MZ-VC 01/29 - Lengpui 739 5 2 7 975 1075 2050 30 MZ-VC 01/30 - Luangpawn 72 2 1 3 162 150 312 31 MZ-VC 01/31 - Lungleng-I 158 2 1 3 301 272 573 32 MZ-VC 01/32 - Lungsei 44 2 1 3 83 85 168 33 MZ-VC 01/33 - Lungsum 92 2 1 3 161 156 317 34 MZ-VC 01/34 - Maite 206 4 1 5 300 279 579 MZ-VC 01 - AIZAWL Name of No. -

Recruitment Notification
No.A.32012 /1/2014 -DTE(SW) GOVERNMENT OF MIZORAM DIRECTORATE OF SOCIAL WELFARE ...... Dated Aizawl, the 14th September, 2018. NOTIFICATION Candidates recommended for appointment for various posts on Contract basis (Co- terminus) under Poshan Abhiyaan, Social Welfare Department (WCD) on the basis of Personal Interview held on 27 th August - 8th September, 2018. I. CONSULTANT (Planning, Monitoring & Evaluation): FATHER/MOTHER'S Sl. No. NAME NAME & ADDRESS 1. Lalfakzuala Dr. Felix Vensanga, College Veng, Aizawl II. CONSULTANT (Health & Nutrition): FATHER/MOTHER'S Sl. No. NAME NAME & ADDRESS 1. R. Lalrinkimi Dr. R. Lalthanga, Hermon Veng, Leitan ‘S’ III. CONSULTANT (Financial Management): FATHER/MOTHER'S Sl. No. NAME NAME & ADDRESS 1. Michael Lalhriatpuia Lalrokuma Pachuau, Mission Vengthlang IV. CONSULTANT (Capacity building & BCC): FATHER/MOTHER'S Sl. No. NAME NAME & ADDRESS 1. RC Vanlalmuani R. Lalnunsanga, Pukpui, Lunglei V. CONSULTANT (Procurement): FATHER/MOTHER'S Sl. No. NAME NAME & ADDRESS 1. Vanlalrosiama Ralte Rohmingliana Ralte, Salem Veng, Aizawl VI. ACCOUNTANT: Sl. FATHER/MOTHER'S NAME No. NAME & ADDRESS 1. Zodinpuii Ralte R. Lalthakima, Ramhlun ‘N’, Aizawl VII. PROJECT ASSOCIATE: Sl. FATHER/MOTHER'S NAME No. NAME & ADDRESS 1. Andy Vanlalduhthlana Zoramchhana (L), Ramhlun ‘N’, Aizawl VIII. DISTRICT COORDINATOR: Sl. FATHER/MOTHER'S PLACE OF POSTING NAME No. NAME & ADDRESS TBC Lawmzuala, 1. Jerry Hmingthansanga DPO Office, Aizawl Laipuitlang, Aizawl Zothanmawia Hnamte, 2. Lalmuansanga Hnamte DPO Office, Champhai Vengthlang ‘N’ , Champhai H. Thangchhuana, 3. Rody H. Vanlalhriatpuii DPO Office, Lawngtlai L-III, Lawngtlai Za Mang, 4. Heibikliana Thangtu CDPO Office, Siaha New Siaha West, Siaha H. Lalramliana, 5. H. Lalrinchhani DPO Office, Mamit Vengthar, Mamit IX. -

Schedule for Selection of Below Poverty Line (Bpl) Families
SCHEDULE-I: SCHEDULE FOR SELECTION OF BELOW POVERTY LINE (BPL) FAMILIES STATE & STATE CODE : MIZORAM 15 NAME OF DISTRICT : CHAMPHAI DISTRICT CODE : 04 NAME OF BLOCK : NGOPA BLOCK CODE : 03 In Thlakhat awmdan Chhungkaw a ST/ Bank RUS Nu/ Village/ Village/ (katcha/ Voter ID Ration hotu chawhruala SC/ Account NO Pa hming Veng Veng Code No semi Card No Card No House in luah# hming pawisa nei/miIn Others No Chhungkaw pucca/ member zat lakluh zat pucca)@ 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1967 Thangliana Khuala (L) 5 1700 Changzawl 10 22 01 11 FDV0198457 10097 ST 97009514505 1968 Lalzamlova Thanliana (L) 3 1700 Changzawl 10 24 01 11 FDV0219915 10068 ST 97000951035 1969 K Lalbiaksanga Lalbiaknunga (L) 2 1700 Changzawl 10 70 01 11 SSZ0022897 10019 ST 97003297000 1970 Lalnunhlima K Zabuanga (L) 7 3500 Changzawl 10 71 01 11 FDV0044826 10046 ST 25034017704 1971 Lalchhanhima Lalduhawma 5 2000 Changzawl 10 72 01 11 FDV0045047 10028 ST 97002524591 1972 Laldanga Ralkapa (L) 2 1700 Changzawl 10 75 01 11 FDV0049297 10030 ST 97002159377 1973 Hrangkima Tlanglawma (L) 6 2000 Changzawl 10 16 01 11 FDV0045039 10011 ST 97003793802 1974 Biakthangsanga Lalchhana 4 3000 Changzawl 10 80 01 11 FDV0062950 10003 ST 25034016438 1975 Lalsawmzuala Sapkhuma 1 2000 Changzawl 10 29 01 10 FDV0044545 10057 ST 97004199294 1976 Lalhmangaiha KT Hranga (L) 1 1500 Changzawl 10 60 01 11 FDV0044776 10034 ST 97004232030 1977 Sapkhuma Vanlalliana (L) 5 1700 Changzawl 10 7 01 11 FDV0045161 10093 ST 25034019553 1978 C Kapmawia Tlanglawma (L) 4 2500 Changzawl 10 16 01 11 FDV0044479 10104 -

Sdeo Khawzawl
No. A.11017/4/2020-DSE(Estt) GOVERNMENT OF MIZORAM DIRECTORATE OF SCHOOL EDUCATION Education Centre, Treasury Square, Aizawl – 796001 Phone : 0389- 2326233Fax : 0389-2317542 Email : [email protected] _______________________________________________________________________________________ Dated Aizawl, the 4th March, 2021 ENGAGEMENT CARD Hmingthantluangi D/o Thangkhuma, Aiduzawl is hereby engaged on Muster Roll basis for the work and for the period mentioned below:- Name of work : Govt. Primary School Teacher (M.R.) Place of work : Govt. P/S, Aiduzawl Period of engagement : 365 days w.e.f. 01.03.2021 - 28.02.2022 Category of labourer : Skilled - II Rate of daily wages : Rs.520/- per day DP &AR(ARW) I.D.No. : ARW/EDN-B/2020-2021/B-241 dt.15.12.2020 Finance concurrence No. : I.D.No FIN(E)378/2020 dt.18.2.2021 Govt. permission No. : A.11011/4/2020-EDN dt. 26.2.2021 Head of account from : 2202 - General Education which expenditure is to 01 - Elementary Education be met 101 - Govt. Primary School (01) - Govt. Primary School/State 00 - (02) - Wages The engagement is purely temporary and the employee shall not be covered by the Regularization Scheme under Govt. of Mizoram and is terminable at anytime without any reason thereof. The employee who has not passed the Teacher Eligibility Test should pass Teacher Eligibility Test as prescribed by NCTE at the earliest. Sd/- JAMES LALRINCHHANA Director of School Education Mizoram : Aizawl. Memo No.A.11017/4/2020-DSE(Estt) : Dated Aizawl, the 4th March, 2021 Copy to :- 1) The Under Secretary to the Govt. of Mizoram, Department of Personnel & Administrative Reforms (ARW). -

Project Staff
Project Staff Thanhlupuia : Research Officer Ruth Lalrinsangi : Inspector of Statistics Lalrinawma : Inspector of Statistics Zorammawii Colney : Software i/c Lalrintluanga : Software i/c Vanlalruati : Statistical Cell Contents Page No. 1. Foreword - (i) 2. Preface - (ii) 3. Message - (iii) 4. Notification - (iv) Part-A (Abstract) 1. Dept. of School Education, Mizoram 2009-2010 at a Glance - 1 2. Number of schools by management - 2 3. Enrolment of students by management-wise - 3 4. Number of teachers by management-wise - 4 5. Abstract of Primary Schools under Educational Sub-Divisions - 5-9 6. Abstract of Middle Schools under Educational Sub-Divisions - 10-16 7. Abstract of High Schools under Educational Districts - 17-18 8. Abstract of Higher Secondary Schools under Educational Districts - 19-23 Part-B (List of Schools with number of teachers and enrolment of students) PRIMARY SCHOOLS: Aizawl District 1.SDEO, AizawlEast - 25-30 2.SDEO, AizawlSouth - 31-33 3.SDEO, AizawlWest - 34-38 4. SDEO, Darlawn - 39-41 5.SDEO, Saitual - 42-43 Champhai District 6.SDEO, Champhai - 44-47 7. SDEO, Khawzawl - 48-50 Kolasib District 8. SDEO, Kolasib - 51-53 9. SDEO, Kawnpui - 54-55 Lawngtlai District 10. EO, CADC - 56-59 11. EO, LADC - 60-64 Lunglei District 12.SDEO, LungleiNorth - 65-67 13.SDEO, LungleiSouth - 68-70 14.SDEO, Lungsen - 71-74 15. SDEO, Hnahthial - 75-76 Mamit District 16. SDEO, Mamit - 77-78 17. SDEO, Kawrthah - 79-80 18.SDEO, WestPhaileng - 81-83 Saiha District 19. EO, MADC - 84-87 Serchhip District 20. SDEO, Serchhip - 88-89 21. SDEO, North Vanlaiphai - 90 22.SDEO, Thenzawl - 91 MIDDLE SCHOOLS: Aizawl District 23.SDEO, Aizawl East - 93-97 24.SDEO, AizawlSouth - 98-99 25. -

Religiosity and Life Satisfaction of Older Persons in Mizoram
RELIGIOSITY AND LIFE SATISFACTION OF OLDER PERSONS IN MIZORAM Jennifer Rohlupuii Department of Social Work Submitted in Partial fulfillment of the requirements of the Degree of Philosophy In Social Work, Mizoram University, Tanhril. Mizoram University April 2019 DECLARATION I, Jennifer Rohlupuii, hereby declare that the subject matter of this thesis is the record of work done by me, that the contents of this thesis did not form basis of the award of any previous degree to me or to the best of my knowledge to anybody else, and that the thesis has not been submitted by me for any research degree in any other University / Institute. This is being submitted to the Mizoram University for the degree of Doctor of Philosophy in Social Work. Date: 24th April, 2019. (JENNIFER ROHLUPUII) Place: Aizawl, Mizoram Research Scholar Department of Social Work (C. DEVENDIRAN) (KANAGARAJ EASWARAN) Professor & Head Professor & Supervisor Department of Social Work Department of Social Work Mizoram University Mizoram University Aizawl – 796004 Aizawl - 796004 i MIZORAM UNIVERSITY April 2019 CERTIFICATE This is to certify that the thesis ‘Religiosity and Life Satisfaction of Older Persons in Mizoram’ submitted by Jennifer Rohlupuii for the award of Doctor of Philosophy in Social Work is carried out under my guidance and incorporates the student’s bonafide research and this has not been submitted for award of any degree in this or any other university or institute of learning. (KANAGARAJ EASWARAN) (C. DEVENDIRAN) Professor & Supervisor Professor & Head Department of Social Work Department of Social Work Mizoram University Mizoram University Aizawl – 796004 Aizawl - 796004 Date: 24th April, 2019. -

The Mizoram.Gazette Publlshed by Autijority , /L " ' Vol
~c1. No. NB 907 ,The Mizoram.Gazette Publlshed by AutIJority , /l " ' Vol. XIX Aizawl Friday 30.1 U990 Agrahayana 9 S.B. 1912 Is!ue No, 48 h, Government of Mlzoram:;, , . -. " PART I') .. 1'~"O"lll!Jents.Postincs. Transfers, Powers. Leave and other. - " Personal Notices and Orders. ,;.* ., NOTIFICATION \ No.A.il20lil/l/90-P.!JRS(1l),u the 21st November.. 1990. In pursuance of Govt. of . hldial Ministry of'.Home Affairs Order No. 14020/55/90-UTS dt. 12. II. 90 or ,.., derin~ transfer/posting in respect of lAS officers: of AGMU Cadre. the Governor of Mizoram in the interest of Public service, is pleased to relieve Pu T.T. Joseph, lAS. (AGMU : 68). Financial Commissioner. Mizoram with effect from the date of handing over :,charge. , ., H. Lal Thlarnuana, Special Secretary to the Govt, of Mizoram, " !!, ,- f; ;, " ., -, .., No.A.19015/19!S9-PAR(R). the 26th November. 1990.•. The Governor of Mizoram J,s pl\'il:sed,to saaction 17 days Earned Leave with effect from 14.11.90 to 30.11.90 ta..Pi K. Lalkimi, -Supdt, Harne Department on private ground under the c.C.S (Leave) Rules, 1972 as amended from time to time. l,Certified, that the ,OlIjcei would have continued to hold the post of Superin- ,l4ndent J;>Ul for lIOI proceeding on leave. 3. Certified that the Officer would have continued to hold the same post and place from where she proceeded on leave and is authorised to draw during leave period the pay and allowances as admissible under the Rules. ..:r,··-l ' "- J;tq;$,\:J90IM84/8$"P~~).{th~ 29th November., IJ90. -

The Mizoram Gazette Published by Authority
The Mizoram Gazette EXTRA ·ORDINARY Published by Authority REGN. NO. N.E.-313 (MZ) Rs. 2/- per Issue VOL. XXX" Aizawl. Wednesday, 26.4.2006, Valsakha 6, S.E. 1928, Issue No. 103 NOTfCATION No. B. 14016/3/02-LAD/VC, the 18th April, 2006. In exercise of the powers co nferred by Section 7(1 )(2), Section 15 and Section 22(1) of the Lusbai Hills District (Village Councils) Act, 1953, as amended from time to time, the Gover- nor of Mizoram is pleased to approve Executive Body of the following Village Councils as shown in the enclosed Annexure within Cha mphai District. R. Sangliankhuma, Addl. Secretary, Local Administration Department. ANNEXURE CHAMPHAI DISTRICT -........------;-------- ----- 1 2 3 4 I4-KHAWBUNG Ale 1. Buang 1. Tinlinga· President 2. Thangchhinga Vice President & Treasurer 3. K. Lalzawngliana Secretary 4. ParlaV\oma Crier Ex-103/20C6 2 1 2 3 4 2. Bungzung J. Lalringzuala President 2. Lalpianfa Vice Pr�sider.t 3. La]zapa Trt:asurer 4. Lalhmingliana Secretary 5. Sangkhuma Crier 3. Bulfekzawl 1. Vanlalrin ga President 2. Chhunkhuma Vice Presifient 3. Chh un tb angvunga Treasurer 4. Laltums2nga Seer etary 5. I. F. }.'Iallga Crier 4. Chawngtui -E' Not yet formed Executive Body 5. Dungtlang 1. H. Laldin gliana President ., .. C. Zalawma Vice President 3. K. LaIthlamuana Treasurer 4. C. Lalzama Secretary 5. Kaprothanga Crier l 6. Farkawn 1. Lalslama President 2. Manghupa Vice President 3. Engm av. ia Treasurer 4. Ljansailova Secretary 5. Lalrawna Crier 7. Hruaikawn 1. C. Hranghleikapa President 2. Sangtinkulha Vice President 3. Sa ngtha n gpuia Treasurer 4 . -

Mizoram Education (Transfer and Posting of School Teachers) Rules
The Mizoral111~Gazette~' EXTRA ORDINARY, ' ' •.. ,- '. ,I Published by Authoritv' REGN. NO. N.E.-313 (MZ) Rs. 2/- per Issue Vol. XXXV Aizawl, W~dnesday, 26.4.2006, Vaihsakha 6, S.E. 1928, Issue No. 104 No .A. 23022/5/2006 EDN, the 12th April, 2006. In exercise of th,e power con• erred by clause (xxxv) of sub-section (2) of sectidh 30 of the Mizoram Educa• tion Act, 2e03 (Act No. 5 of .2003), the Governor of Mizoram" is pleased to make the [allowing rules, namely :- . 1. SnORT TITLE? EXTENT AND COMMENCEMENT : (1) These Rules may be called the Mizoram Education (Transfer and . Posting of School Teachers) Rules, 2006, (2) It shall extend to the whole of Mizoram in respect of Elementary ,Educdtion and Secondary£ducation, except Chakllla Autonomous District, Lai Autonomou3 District and Mara Autohomous Dis• trict in respect of Elementary Education 'only. (3) These rul es shall com::: into force from til~ date of notificati on in the Mizoram Gazette. 2. IN THESE RULES, UNLESS THE CONTEXT OTHERWISE REQUIRES : ;- (1')' "Act" means MizoramEducati~n Act, 2003 (Act No. 5 of 2003) (2) ""Appropriate AuthoJity" shall mean the authority.as specified in Rule 9; (3) "District" means the jurisdiction area prescribed by tb.e Government of District Education Officer; (4) "Elementary School" means Primary Schools and Middle Schools~ (5) "Government" means the Government of Mizoram. (€i) "Sanctioned strellgth'>"me'<lDSth.t' number of posts 'of teachers as a 'particular School as Sanctiolled by the Government., -', , (-7) "Schedule" m6anl~a Schedul~ appended .,to these Rule·s; -l"fl' Ex-l04/2006 2 (8) "Secondary School" means High Schools and Higher Secondary Schools. -

SDEO CHAMPHAI Sl
SELECTED CANDIDATES FOR TEMPORARY ENGAGEMENT OF MIDDLE SCHOOL HINDI TEACHERS UNDER CSS WITH PLACE OF POSTING, 2020-2021 UNDER SDEO CHAMPHAI Sl. Name of Name and Address Qualification Remarks Name Of School Place of Posting No Training Hindi General Lalkhawngaihkimi Praveen Hindi Shikshan 1 D/o Nghakliana HSSLC Govt Vangchhia M/S SDEO Champhai (Mizoram) Diploma Khuangthing H/No V-57 Jenny Lalrinmawii Praveen Hindi Shikshan 2 D/o B.Chhuahkhama HSSLC Govt Hmunhmeltha M/S SDEO Champhai (Mizoram) Diploma Hmunhmeltha, Champhai Lynda Zomuanpuii Praveen Hindi Shikshak Govt Middle School,New 3 D/o M.Liana HSSLC SDEO Champhai (Mizoram) Diploma Hruaikawn Bungkawn AW-31(A) C.Zalianpari Praveen 4 D/o C.Lalmuana B.A Parangat Govt Mualkawi M/S SDEO Champhai (Mizoram) Mualkawi,Champhai District Lalhriattiri D/o Kepliana Praveen Hindi Shikshak 5 HSSLC Thekpui UPS SDEO Champhai Farkawn,Theirekawn (Mizoram) Diploma Champhai Dist. Nangkapliana Praveen Hindi Shikshak 6 S/o SB Sawngkhawzama HSSLC Govt. Border M/S, Sesih SDEO Champhai (Mizoram) Diploma Ramhlun South, H/No Y-106 S.Kapkhandova Praveen Hindi Shikshan 7 S/o S.Hauzanang B.A Govt Melbuk M/S SDEO Champhai (Mizoram) Praveen Melbuk, Champhai F.Zodinpuii Praveen Hindi Shikshak 8 D/o Tlangchhana HSSLC Govt Khuangleng M/S SDEO Champhai (Mizoram) Diploma Khuangleng James Lalmalsawma S/o Siamhnuna Praveen Hindi Shikshak 9 HSSLC Presby.Eng.School,Hnahlan SDEO Champhai Durtlang Leitan, Ramthar (Mizoram) Diploma H/No LV/C-74 Vungsailiani Praveen Hindi Shikshak 10 D/o Ngindothanga HSSLC Govt Leisenzo M/S SDEO -

Government of India Ministry of Tourism Rajya Sabha
GOVERNMENT OF INDIA MINISTRY OF TOURISM RAJYA SABHA UNSTARRED QUESTION NO.318 ANSWERED ON 15.09.2020 VILLAGE TOURISM IN THE COUNTRY 318. SHRI RAKESH SINHA: Will the Minister of TOURISM be pleased to state: (a) whether the Ministry has contemplated any plan extending the tourism to Indian Villages which reflects India's true cultural and social Identity; (b) whether the Ministry has contemplated and mooted a plan for "Village Tourism"; (c) if so, the number of villages that are included for "Village Tourism" and the details of their locations; and (d) if not, whether the Ministry will discuss and promote the idea of "Village Tourism" in India? ANSWER MINISTER OF STATE FOR TOURISM (INDEPENDENT CHARGE) (SHRI PRAHLAD SINGH PATEL) (a) to (d): Yes, Sir. The Ministry of Tourism has launched the Swadesh Darshan Scheme for Integrated Development of theme-based Tourist Circuits for development of tourism infrastructure including last mile connectivity in the country. Recognizing the potential of rural tourism in the country, the Ministry has identified Rural Circuit as one of fifteen thematic circuits identified for development under this scheme. The objectives of the Swadesh Darshan scheme include creating employment through active involvement of local communities and promoting community-based development and pro-poor tourism approach. Based on the above criteria, the Ministry has sanctioned following 2 projects total amounting to Rs.125.02 Crores for development of Rural Circuits under the Swadesh Darshan Scheme which are under different stages of implementation: - i. In the State of Bihar, during FY 2017-18, the project “Development of Bhitiharwa - Chandrahia - Turkaulia” amounting to Rs.44.65 Crores.