Lepidoptera-Specific Insecticide Used to Suppress Gypsy Moth Outbreaks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Survey of Four Longleaf Pine Preserves
A SURVEY OF THE MOTHS, BUTTERFLIES, AND GRASSHOPPERS OF FOUR NATURE CONSERVANCY PRESERVES IN SOUTHEASTERN NORTH CAROLINA Stephen P. Hall and Dale F. Schweitzer November 15, 1993 ABSTRACT Moths, butterflies, and grasshoppers were surveyed within four longleaf pine preserves owned by the North Carolina Nature Conservancy during the growing season of 1991 and 1992. Over 7,000 specimens (either collected or seen in the field) were identified, representing 512 different species and 28 families. Forty-one of these we consider to be distinctive of the two fire- maintained communities principally under investigation, the longleaf pine savannas and flatwoods. An additional 14 species we consider distinctive of the pocosins that occur in close association with the savannas and flatwoods. Twenty nine species appear to be rare enough to be included on the list of elements monitored by the North Carolina Natural Heritage Program (eight others in this category have been reported from one of these sites, the Green Swamp, but were not observed in this study). Two of the moths collected, Spartiniphaga carterae and Agrotis buchholzi, are currently candidates for federal listing as Threatened or Endangered species. Another species, Hemipachnobia s. subporphyrea, appears to be endemic to North Carolina and should also be considered for federal candidate status. With few exceptions, even the species that seem to be most closely associated with savannas and flatwoods show few direct defenses against fire, the primary force responsible for maintaining these communities. Instead, the majority of these insects probably survive within this region due to their ability to rapidly re-colonize recently burned areas from small, well-dispersed refugia. -

And Lepidoptera Associated with Fraxinus Pennsylvanica Marshall (Oleaceae) in the Red River Valley of Eastern North Dakota
A FAUNAL SURVEY OF COLEOPTERA, HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH FRAXINUS PENNSYLVANICA MARSHALL (OLEACEAE) IN THE RED RIVER VALLEY OF EASTERN NORTH DAKOTA A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By James Samuel Walker In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Entomology March 2014 Fargo, North Dakota North Dakota State University Graduate School North DakotaTitle State University North DaGkroadtaua Stet Sacteho Uolniversity A FAUNAL SURVEYG rOFad COLEOPTERA,uate School HEMIPTERA (HETEROPTERA), AND LEPIDOPTERA ASSOCIATED WITH Title A FFRAXINUSAUNAL S UPENNSYLVANICARVEY OF COLEO MARSHALLPTERTAitl,e HEM (OLEACEAE)IPTERA (HET INER THEOPTE REDRA), AND LAE FPAIDUONPATLE RSUAR AVSESYO COIFA CTOEDLE WOIPTTHE RFRAA, XHIENMUISP PTENRNAS (YHLEVTAENRICOAP TMEARRAS),H AANLDL RIVER VALLEY OF EASTERN NORTH DAKOTA L(EOPLIDEAOCPTEEAREA) I ANS TSHOEC RIAETDE RDI VWEITRH V FARLALXEIYN UOSF P EEANSNTSEYRLNV ANNOICRAT HM DAARKSHOATALL (OLEACEAE) IN THE RED RIVER VAL LEY OF EASTERN NORTH DAKOTA ByB y By JAMESJAME SSAMUEL SAMUE LWALKER WALKER JAMES SAMUEL WALKER TheThe Su pSupervisoryervisory C oCommitteemmittee c ecertifiesrtifies t hthatat t hthisis ddisquisition isquisition complies complie swith wit hNorth Nor tDakotah Dako ta State State University’s regulations and meets the accepted standards for the degree of The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of University’s regulations and meetMASTERs the acce pOFted SCIENCE standards for the degree of MASTER OF SCIENCE MASTER OF SCIENCE SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: SUPERVISORY COMMITTEE: David A. Rider DCoa-CCo-Chairvhiadi rA. -

Fauna of Sable Island by Barry Wright Errors and Omissions
Nova Scotia ~~,.. Curatorial Report Number 68 Nova Scotia Museum ..,~, 1747 Summer Street The Halifax I Nova Scotia I Canada Department of B3 H 3A6 Education Fauna of Nova Scotia Museum Complex Sable Island By Barry Wright October 1989 Curatorial Report no. 68 The Fauna of Sable Island by Barry Wright Errors and Omissions Insert the following at the bottom of page 74 Gasterosteus aculeatus Linnaeus Threespine Sticklebacks were collected by David Marcogliese in Pond number 7 in August 1990. The Fourspine Sticklebacks taken in East Pond. (Garside 1969) were checked by John Gilhen and found to be identified correctly. Insert the following references on Page 92. Erskine, J.S., 1954. The Ecology of Sable Island, 1952. Proc. N.S.lnst. Sd., 23: 120-145. Farquhar, J.A., 1947. Extracts from the Journal of the late Captain Farquhar: His stay on Sable Island. Coli. N.S. Hist. Soc., 27: 100-124. Farquhar, J.A., 1980. Farquhar's Luck. Petheric Press Ltd., Halifax, N.S., 188 pp. Fernald, M.L, & St. John, H., 1914. The varieties of Hieraceum scabrum. Rhodora, 16:181-183. Freedman, B., Catling, P.M. and Lucas, Z., in Taylor, R.B., 1982. The Vegetation of Sable Island, Nova Scotia. Report on Terrain Management and Biological Studies on Sable Island, 1981, Prepared for the Sable Island Environmental Advisory Committee. 71 pp. Ganong, W .F., 1908. The Description and Natural History of the Coasts of North America (Acadia) by Nicholas Denys. The Champlain Society, Toronto. p 207. Garside, E.T., 1969. Distribution of Insular Fishes of Sable Island, Nova Scotia.]. -

Light-Trap Catches of Moths Within and Above the Canopy of a Northeastern Forest
Journal of the Lepidopterists' Society 50(1), 1996, 21-45 LIGHT-TRAP CATCHES OF MOTHS WITHIN AND ABOVE THE CANOPY OF A NORTHEASTERN FOREST A, W, THOMAS Natural Resources Canada, Canadian Forest SelVice-Fredericton, Fredericton, New Brunswick E3B 5P7, Canada ABSTRACT. A catch of 10,991 moths, comprising 311 species in 15 selected families, was identified from two 22-watt blacklight traps operating for 29 nights between 21 June and 30 July 1990. Nightly catches ranged from 4 to 824 individuals per trap. In the within canopy site, 6,088 individuals of 255 species were identified, whereas in the above-canopy site, 4,903 individuals of 269 species were identified. There were 213 species common to both sites. The coefficient of similarity (of species) between sites was 0.862 (Morisita Hom index). The percentage complementarity between sites was 31..5 (Marczewski-Stein haus distance). Moths in the family Noctuidae dominated the identified catches, accounting for 43.5% of the species and 36.6% of the individuals within the canopy, and 49.4% of the species and 52.4% of the individuals above the canopy. Moths in the family Geometridae were the next most common identified group, forming 33% of individuals in the canopy and 26% of individuals above the canopy. Members of no other Single family formed more than 8% of the identified individuals. Several non-tree-feeding species and four known migrants were collected only above the canopy. Each trap's nightly catch was separated into 30-minute sequential samples, 16/night, between 2130-0530 h ADT. Individuals were trapped all night, but on average catches peaked at 2300-2330 h, two hours after sunset. -

Plum Island Biodiversity Inventory
Plum Island Biodiversity Inventory New York Natural Heritage Program Plum Island Biodiversity Inventory Established in 1985, the New York Natural Heritage NY Natural Heritage also houses iMapInvasives, an Program (NYNHP) is a program of the State University of online tool for invasive species reporting and data New York College of Environmental Science and Forestry management. (SUNY ESF). Our mission is to facilitate conservation of NY Natural Heritage has developed two notable rare animals, rare plants, and significant ecosystems. We online resources: Conservation Guides include the accomplish this mission by combining thorough field biology, identification, habitat, and management of many inventories, scientific analyses, expert interpretation, and the of New York’s rare species and natural community most comprehensive database on New York's distinctive types; and NY Nature Explorer lists species and biodiversity to deliver the highest quality information for communities in a specified area of interest. natural resource planning, protection, and management. The program is an active participant in the The Program is funded by grants and contracts from NatureServe Network – an international network of government agencies whose missions involve natural biodiversity data centers overseen by a Washington D.C. resource management, private organizations involved in based non-profit organization. There are currently land protection and stewardship, and both government and Natural Heritage Programs or Conservation Data private organizations interested in advancing the Centers in all 50 states and several interstate regions. conservation of biodiversity. There are also 10 programs in Canada, and many NY Natural Heritage is housed within NYS DEC’s participating organizations across 12 Latin and South Division of Fish, Wildlife & Marine Resources. -

Effects of Standard Management Practices On, and Faunistics of Native Prairies
Conservation Biology Research Grants Program Division of Ecological Services Minnesota Department of Natural Resources EFFECTS OF STANDARD MANAGEMENT PRACTICES ON, AND FAUNISTICS OF NATIVE PRAIRIES: A study of three sites in western Minnesota. Report submitted to: Minnesota and Wisconsin Departments of Natural Resources, The Nature Conservancy, and the R. J. Kose foundation. David Rider, Systematic Entomologist Gerald Fauske, Research specialist Paul Tinerella, Graduate student Department of Entomology North Dakota State University Fargo, ND May 2000 ABSTRACT From 1995 through 1999 an ongoing study was conducted to examine the effects of standard prairie management practices (burning, grazing, haying) and unmanaged prairie, with regard to arthropod communities. Additional objectives were to gather baseline data on the arthropod species present on remnant prairies of western Minnesota, to identify rare species found on theses sites, and if possible, to determine prairie indicator species which might be used to identify dry, mesic, or wet prairies types in a manner similar to the plant indicator species used in botanical studies. At present, nearly 35,000 insect specimens have been pinned, labeled, and identified representing more than 750 species. As additional material is processed and identified, we expect the number of species found on these sites to more than double, perhaps even triple. This report provides a summary of work completed as of May, 2000, and includes species lists, information on species distributions (state records and extensions of known species ranges), presence of rare or state endangered species on these prairies, diversity indices for prairie sites, types, and management practices, and management histories of the study areas. -
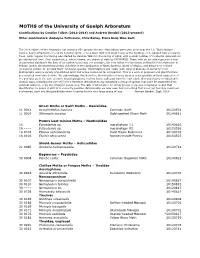
Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Earley, Fiona Reid, Mike Kent The 2018 edition of the Arboretum list contains 851 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent a first documented sighting if the date of an earlier record was not available. We now follow the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a group of species that can't be separated from external features, or by identifying to genus only. -

Lepidoptera: Noctuidae) from Pakistan with Cladistic Analysis
BIODIVERSITY AND FAUNISTIC STUDIES OF THE SUB-FAMILY NOCTUINAE (LEPIDOPTERA: NOCTUIDAE) FROM PAKISTAN WITH CLADISTIC ANALYSIS SHAHEEN NAZ RESEARCH CARRIED OUT AT THE FEDERAL URDU UNIVERSITY OF ARTS, SCIENCES AND TECHNOLOGY, GULSHAN-E- IQBAL, KARACHI DEPARTMENT OF ZOOLOGY KARACHI-75300 PAKISTAN 2011 i BIODIVERSITYAND FAUNISTIC STUDIES OF THE SUB-FAMILY NOCTUINAE (LEPIDOPTERA: NOCTUIDAE) FROM PAKISTAN WITH CLADISTIC ANALYSIS BY SHAHEEN NAZ M.Sc.(Kar.), B. Ed. FEDERAL URDU UNIVERSITY OF ARTS SCIENCES AND TECHNOLOGY, GULSHAN-E-IQBAL, KARACHI. KARACHI-75300, PAKISTAN 2011 ii BIODIVERSITYAND FAUNISTIC STUDIES OF THE SUB-FAMILY NOCTUINAE (LEPIDOPTERA: NOCTUIDAE) FROM PAKISTAN WITH CLADISTIC ANALYSIS By SHAHEEN NAZ M.Sc.(Kar.), B. Ed. A thesis submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in the Faculty of Science Federal Urdu University of Arts, Sciences and Technology, Karachi RESEARCH CARRIED OUT AT THE FEDERAL URDU UNIVERSITY OF ARTS, SCIENCES AND TECHNOLOGY, GULSHAN-E- IQBAL, KARACHI DEPARTMENT OF ZOOLOGY KARACHI-75300 PAKISTAN 2011 iii This thesis entitled“BIODIVERSITY AND FAUNASTIC STUDIES OF THE SUB-FAMILY NOCTUINAE (LEPIDOPTERA: NOCTUIDAE) FROM PAKISTAN WITH CLADISTIC ANALYSIS” by Shaheen Naz is accepted its present form by the Department of Zoology, Federal Urdu University of Arts, Sciences and Technology, as satisfying the requirements of the degree of Doctor of Philosophy. Supervisor: Co-Supervisor: Dr. Riffat Ameer Prof. Dr. Syed Kamaluddin Government Degree Girls Department of Zoology College, 11-B North Karachi. Federal Urdu University Karachi. of Arts, Sciences and Technology,Gulshan-e- Iqbal, Karachi. iv CERTIFICATE It is to certify that the results, diagrams, all the data and description, used in this thesis, entitled “Biodiversity and Faunistic studies of the Sub-family Noctuinae (Lepidoptera: Noctuidae) from Pakistan with cladistic analysis” submitted by Shaheen Naz, are not copied and the whole manuscript and diagrams are made by himself. -

(Lepidoptera) Inhabiting the Funk Bottoms Wildlife Area, Wayne and Ashland Counties, Ohio1
Survey of the Moths (Lepidoptera) Inhabiting the Funk Bottoms Wildlife Area, Wayne and Ashland Counties, Ohio1 R. N. WILLIAMS, R. W. RINGS, M. S. ELLIS, AND D. S. FICKLE, Department of Entomology, Ohio Agricultural Research and Development Center, The Ohio State University, 1680 Madison Avenue, Wooster, OH 44691 ABSTRACT. In 1995, the Funk Bottoms Wildlife Area was the subject of an ongoing series of insect surveys intended to establish benchmark information on arthropod diversity of wetlands in northeast Ohio. This article concentrates on the moths which were collected at ultraviolet light traps within the Funk Bottoms Wildlife Area. A companion report will follow focusing on the Coleoptera along with several orders of aquatic insects. 3252 specimens were identified to 306 species in 19 families. These species are classified as follows: Abundant = 34; Locally Abundant = 1; Common = 257; Locally Common = 2; Uncommon = 10; Rare = 1; and Special Interest = 1. OHIO J. SCI. 97 (3): 34-39, 1997 INTRODUCTION Army Corps of Engineers. All the land behind the dam, The Funk Bottoms Wildlife Area was founded in 1991 below an elevation of 294 m, is under flood easement. with the initial purchase of land. The fact that this Wild- As a result, 3560 ha, including the Funk Bottoms Wildlife life Area is so new only magnifies the importance of Area, make up this wetland. There is relatively little area understanding the species existing there and what effect (around 80 ha) of permanently wet soils (marsh) within environmental changes might have upon them. This the easement area. However, hundreds to thousands of Wildlife Area currently consists of 467 ha which will be hectares may be inundated for periods of days to several expanded as funds and lands become available. -

Cutworm Pests of Crops on the Canadian Prairies
Cutworm Pests of Crops on the Canadian Prairies IDENTIFICATION AND MANAGEMENT FIELD GUIDE Cutworm Pests of Crops on the Canadian Prairie: Identification and Management Field Guide Cover photo: Armyworm cutworm larva and damage, Mike Dolinski, [email protected] © Her Majesty the Queen in Right of Canada, represented by the Minister of Agriculture and Agri-Food Canada (2017). Electronic version available at www.publications.gc.ca Catalogue No. A59-42/2017E-PDF ISBN 978-0-660-08051-2 AAFC No. 12614E This publication may be cited as follows: Floate, K.D. 2017. Cutworm pests on the Canadian Prairies: Identification and management field guide. Agriculture and Agri-Food Canada, Lethbridge, Alberta. Paru également en français sous le titre Guide d’identification des vers-gris ravageurs des cultures dans les Prairies canadiennes et mesures de lutte applicables. For more information, reach us at www.agr.gc.ca or call us toll free at 1-855-773-0241 Acknowledgements Thank you to Dr. John Gavloski and Hugh Philip for comments on earlier drafts and to Dr. Jeremy Hummel for his thorough technical review of the final version. Erl Svendsen handled the editing, proofing, sourcing and selecting of images and initial layout design, while Chennoa Tracey did most of the heavy lifting on the graphic design of this guide. Thank you also to the many photographers who made their images available for this publication. Funding for preparation of this publication was provided by the Canola Council of Canada (CARP Project No. 2012-1) with contributions from the Alberta Canola Producers Commission, Saskatchewan Canola Development Commission (SaskCanola) and the Manitoba Canola Growers Association in partnership with the Alberta Crop Industry Development Fund. -

Proceedings of the Entomological Society of Washington
PROC. ENTOMOL. SOC. WASH. 82(4), 1980, pp. 576-586 'NOCTVA C-NIGRVM' IN EASTERN NORTH AMERICA, THE DESCRIPTION OF TWO NEW SPECIES OF XESTIA HUBNER (LEPIDOPTERA: NOCTUIDAE: NOCTUINAE) John G. Franclemont Department of Entomology, Cornell University, Ithaca, New York 14853. Abstract. —The species formerly called Amatlws c-ni^rum (L.) or Noctua c-nigriim, the "spotted cutworm" of economic literature, is shown to be two species in North America, a smaller species, Xestia adela, n. sp., and a larger species, Xestia dolosa, n. sp.; adela is the species more like the Eurasian c-nii^ntm. The two species have different distributions, but occur together over a large area of southeastern Canada and northeastern United States; adela occurs in most of the Canadian provinces, Alaska, the con- terminous United States, and Mexico, whereas dolosa is known only from southeastern Canada and the northeastern and north central United States. This study had its inception in the fall of 1969 when Andre Comeau brought me two lots of three moths each of a small and large species, both then called Ainathes c-nii^rum (Linnaeus, 1758), that had been taken at Geneva, New York in sex pheromone traps, one set baited with cis-7-tet- radecenyl acetate and the other with trans-7-tetradecenyl acetate (Roelofs and Comeau, 1970). In 1946 I had decided that specimens of Amathes crassipuncta (Wileman and South, 1928) that I thought were c-nii^rum when collected in Baguio, Mountain Province, Luzon, Philippines, during May and June of 1945 were different from both European and New York State specimens of ""c-ni- iirum.'" I also decided that the New York species, I then had only specimens of the "large species," was distinct from the central European species. -

Camp Edwards Integrated Fire Management Plan (IFMP)
INTEGRATED FIRE MANAGEMENT PLAN CAMP EDWARDS TRAINING SITE MASSACHUSETTS ARMY NATIONAL GUARD This Integrated Fire Management Plan (IFMP) meets all requirements as described in the Army Wildland Fire Policy Guidance and references therein, Army Regulation 200-3 (Natural Resources-Land, Forest and Wildlife Management), Army Regulation 420-90 Fire and Emergency Services, and the Executive Summary of this document. Furthermore, the undersigned do hereby agree to cooperate in the implementation of the Camp Edwards IFMP. ________________________________________________Date:___________________ Adjutant General of Massachusetts Office of the Adjutant General Massachusetts National Guard ________________________________________________Date:___________________ Director of Facilities and Engineering Massachusetts Army National Guard ________________________________________________Date:___________________ Environmental Program Manager Massachusetts Army National Guard ________________________________________________Date:___________________ Commander, Camp Edwards Training Center Massachusetts Army National Guard Camp Edwards, Massachusetts ________________________________________________Date:___________________ Director of Plans, Operations, and Training Massachusetts Army National Guard Camp Edwards, Massachusetts ________________________________________________Date:___________________ Natural Resource Manager Massachusetts Army National Guard Camp Edwards, Massachusetts i INTEGRATED FIRE MANAGEMENT PLAN CAMP EDWARDS TRAINING SITE MASSACHUSETTS