Lectins: Production and Practical Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ethnomedicinal Survey of Uri, Kashmir Himalaya
Indian Journal of Traditional Knowledge Vol. 3(4), October 2004, pp. 351-357 Ethnomedicinal survey of Uri, Kashmir Himalaya Z S Khan, A A Khuroo* and G H Dar Centre of Plant Taxonomy (COPT), University of Kashmir, Srinagar 190 006, Jammu & Kashmir E-mail: [email protected] Received 6 October 2003; revised 15 April 2004 In the contemporary global milieu, the documentation of the biological resources and the associated indigenous knowledge existing within a country has assumed highest priority. The present paper records ethnomedicinal value of 27 plant species belonging to 20 families, in vogue, from the study area. Each plant species included, contains information regarding crude drug preparation and its method of use. Such documentation would be helpful in terms of com- mercial production of drugs, readily accessible health care to larger population, sustainable use and above all, safeguard from bio-piracy. Keywords: Indigenous knowledge, Medicinal plants, Ethnomedicine, Kashmir. IPC Int. Cl.7: A61K35/78, A61P1/04, A61P1/10, A61P11/10, A61P13/00, A61P13/02, A61P15/06, A61P15/14, A61P17/02, A61P17/10, A61P19/02, A61P27/02, A61P27/12, A61P33/10. From the very earliest days of civiliza- About 70% of the identified medicinal tion, mankind has turned to plants for plants of Indian Himalaya are exposed to healing, a tradition that has survived the destructive harvesting6. Recently our arrival of modern medicine and found country enacted a number of legisla- new strength at the end of 20th century1. tions7-9, in compliance with CBD and Even today, 80% of the world’s popula- WTO, in order to prevent the unfair ex- tion relies on traditional plant medicine2. -

LOST HORIZONS 2018 CATALOGUE Table Of
WELCOME TO LOST HORIZONS 2018 CATALOGUE Table of Contents Great Plants/Wonderful People . 15. Nomenclatural Notes . 15. Some History . 16. Availability . .17 . Recycle . 17 Location . 17 Hours . 18 Note on Hardiness . 18. Gift Certificates . 18. Lost Horizons Garden Design, Consultation, and Construction . 19. Understanding the catalogue . 19. References . 20. Catalogue . 21. Perennials . .21 . Acanthus . .21 . Achillea . .21 . Aconitum . 21. Actaea . .22 . Adenophora . 23. Agastache . .23 . Ajuga . 23. Alchemilla . 24. Allium . .24 . Alstroemeria . .25 . Alyssum . 25. Amsonia . 25. Anemone . .26 . Anemonella . .27 . Anemonopsis . 27. Angelica . 28. Anthemis . .28 . Anthericum . .28 . For more info go to www.losthorizons.ca - Page 1 Aquilegia . 29. Arabis . .29 . Aralia . 29. Arenaria . 30. Arisaema . .30 . Arisarum . .31 . Armeria . .31 . Armoracia . .32 . Artemisia . 32. Arum . .32 . Aruncus . .33 . Asarum . .33 . Asclepias . .33 . Asparagus . .34 . Asphodeline . 34. Aster . .34 . Astilbe . .35 . Astilboides . 35. Astragalus . .36 . Astrantia . .36 . Aubrieta . 37. Baptisia . .37 . Begonia . .38 . Bergenia . 38. Bletilla . 38. Boehmeria . .39 . Bolax . .39 . Brunnera . .39 . Buphthalmum . .40 . Cacalia . 40. Caltha . 41. Campanula . 41. For more info go to www.losthorizons.ca - Page 2 Cardamine . .42 . Cardiocrinum . 42. Caryopteris(Perennial) . 43. Cassia . 43. Caulophyllum . .43 . Centaurea . 44. Cephalaria . .44 . Chelone . .44 . Chelonopsis . 45. Chiastophyllum . .45 . Chloranthus . .45 . Chrysanthemum . .. -

The Cummins Garden
Foreword For a few weeks this summer, I found it Time helps. But there's nothing like need to impossible to g arden. A tremendous lethargy loosen the grip on sloth. Now with the short crept through me and the simple joys of days of fall upon me, I've begun to pick up working with plants and the soil ceased for the pace, re-covering the greenhouse, a time. What did remain, though, was the collecting seed, planting those innumerable same sense of wonder on seeing the first pots which seem to gather at the doorstep of bloom on a new plant or new seedlings in a every collector. Just maybe, I'll be ready to seed pot which has been with me for the 30 move inside when the inevitable winter plus years I've been gardening. During the rains settle in for good. haitus of spirit, I reflected on the continuum This is the last issue of the Bulletin which I which make a gardener, from the first brush will be editing. I would like to thank those with nature as a child to the more sobering of you who have provided very special help awareness that gardens and plants (as well during the past year. as people) need care and nurturing to be at their best. Ted Marston On the Cover: Calthapalustris, from a copperplate by Abraham Munting first published in 1696 Published quarterly by the AMERICAN ROCK GARDEN SOCIETY, a tax-exempt, non-profit organization incorpo• rated under the laws of the state of New Jersey. -

SILICO DISCOVERY of NATURAL LEAD HITS from the GENUS of Arisaema AGAINST HUMAN RHINO VIRUS
IN- SILICO DISCOVERY OF NATURAL LEAD HITS FROM THE GENUS OF Arisaema AGAINST HUMAN RHINO VIRUS Kamal Kant, Uma Ranjan Lal, Manik Ghosh* Department of Pharmaceutical Sciences and Technology, Birla Institute of Technology, Mesra, Ranchi, Jharkhand (835215), INDIA *Corresponding Author’s E-mail: [email protected] Tel.: + 916512276247; Fax: + 916512275290 ABSTRACT Human rhino viruses (HRVs) serve as an imperative precursor for frequent cases of the common cold among children worldwide. An in-silico molecular docking attempt was made of some chief phytochemical entities (Arisaema plant species) as inhibitors of HRVs with selective therapeutic target action and minimal side effects. Around 60 phytoconstituents of different Arisaema species were docked against HRV receptor (PDB: 2XYA). Binding conformers of test ligands were compared with internal ligand. Finally, Syringaresinol 4'-O-β-D-glucopyranoside (glide score: - 10.86), Rutin (glide score: -9.04) & Apigenin-6,8-di-C-β-D-glucopyranoside (glide score: -7.88) have resulted in most promising hits which can be further act as an effective template to experimentally validate or further designed their choosy and budding analogue agents against HRVSs. Keywords: Arisaema, human rhino virus, docking INTRODUCTION Presently, it is very imperative to mitigate the challenge of developing an antiviral drug for treatment of common cold. Common cold is a disease which is being caused by rhino viruses due to lack of specific treatment against this virus. Although, randomized therapy possess some better therapeutic effect but at the same time patients may also suffer from some adverse effects. The symptom includes some cholinergic effect on the peripheral nervous system followed by nasal stuffiness, sneezing, cough & throat infections. -

Exploring Patterns of Phytodiversity, Ethnobotany, Plant Geography and Vegetation in the Mountains of Miandam, Swat, Northern Pakistan
EXPLORING PATTERNS OF PHYTODIVERSITY, ETHNOBOTANY, PLANT GEOGRAPHY AND VEGETATION IN THE MOUNTAINS OF MIANDAM, SWAT, NORTHERN PAKISTAN BY Naveed Akhtar M. Phil. Born in Swat, Khyber Pakhtunkhwa, Pakistan A Dissertation Submitted in Partial Fulfillment of the Requirements for the Academic Degree of Doctor of Philosophy (PhD) in the Georg-August-University School of Science (GAUSS) under Faculty of Biology Program Biodiversity and Ecology Georg-August-University of Göttingen Göttingen, 2014 ZENTRUM FÜR BIODIVERSITÄT UND NACHHALTIGE LANDNUTZUNG SEKTION BIODIVERSITÄT, ÖKOLOGIE UND NATURSCHUTZ EXPLORING PATTERNS OF PHYTODIVERSITY, ETHNOBOTANY, PLANT GEOGRAPHY AND VEGETATION IN THE MOUNTAINS OF MIANDAM, SWAT, NORTHERN PAKISTAN Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität Göttingen Vorgelegt von M.Phil. Naveed Akhtar aus Swat, Khyber Pakhtunkhwa, Pakistan Göttingen, 2014 WHEN WE ARE FIVE AND THE APPLES ARE FOUR MY MOTHER SAYS “I DO NOT LIKE APPLES” DEDICATED TO My Mother Supervisor: Prof. Dr. Erwin Bergmeier Albrecht-von-Haller-Institute ofPlant Sciences Department of Vegetation & Phytodiversity Analysis Georg-August-University of Göttingen Untere Karspüle 2 37073, Göttingen, Germany Co-supervisor: Prof. Dr. Dirk Hölscher Department of Tropical Silviculture & Forest Ecology Georg-August-University of Göttingen Büsgenweg 1 37077, Göttingen, Germany Table of Contents Acknowledgements ........................................................................................................................................ -

Vascular Plants, Scott Christian College, Nagercoil, Tamilnadu, India
Science Research Reporter, 5(1):36-66, (April - 2015) © RUT Printer and Publisher Online available at http://jsrr.net ISSN: 2249-2321 (Print); ISSN: 2249-7846 (Online) Research Article Vascular Plants, Scott Christian College, Nagercoil, Tamilnadu, India Thankappan Sarasabai Shynin Brintha, James Edwin James and Solomon Jeeva* Scott Christian College (Autonomous), Research Centre in Botany, Nagercoil – 629 003, Tamilnadu, India *[email protected] Article Info Abstract Received: 10-03-2015, The biodiversity and ecosystem functioning of urban environments is receiving increasing attention from ecologists. In this context we inventoried the vascular Revised: 27-03-2015, plant diversity of Scott Christian College campus which harbours part of the Accepted: 01-04-2015 natural vegetation of Nagercoil city, Tamilnadu, India. A total of 670 plant species including 651 flowering plants and 19 non-flowering plants, belonging Keywords: to 450 genera and 125 families were enumerated. The family Poaceae was the most species-diverse (60), followed by Euphorbiaceae (37), Fabaceae (35), Vascular Plants, Biodiversity, Acanthaceae (30), Asteraceae (27), Rubiaceae (24), Araceae (21), Malvaceae Ecosystem, Flowering plants, (20), Caesalpiniaceae (19), Amaranthaceae and Apocynaceae (17 each), Conservation Moraceae (16), Convolvulaceae and Mimosaceae (14 each) Verbenaceae (13), Cucurbitaceae (11), Bignoniaceae, Solanaceae and Asclepiadaceae (10 each), the other families sharing the rest of the species. The results of this study provide insights into the importance of urban green space and greatly help in urban conservation planning and management. INTRODUCTION and further reflects both anthropogenic and natural Biodiversity reflects variety and variability disturbances (Pollock, 1997; Ward, 1998). within and among living organisms, their Therefore, floristic characteristics and biodiversity associations and habitat-oriented ecological patterns are often influenced by environmental complexes. -

Indigenous Utilization of Plant Biodiversity in Malakkheil-Kotkay
Pak. j. sci. ind. res. Ser. B: biol. sci. 2019 62B(1) 15-23 Indigenous Utilization of Plant Biodiversity in Malakkheil-Kotkay Village, District Shangla, Pakistan Shafqat Ali Khan, Fazal Hadi*, Muhammad Ibrar and Ulfat Samreen Department of Botany, University of Peshawar, Peshawar, Pakistan (received March 13, 2017; revised November 2, 2017; accepted November 29, 2017) Abstract. The present study was carried out in village Malakkheil-Kotkay, District Shangla, Pakistan that revealed 84 plants belonging to 51 families utilized for the treatment of different diseases. Rosaceae was leading family with 10 species followed by Asteraceae and Lamiaceae with 7 species each, Araceae and Polygonaceae with 3 species each and Amaranthaceae with 2 species. The remaining families had one species each. The life form spectra showed 31 (39.74%) therophytes, 17 (21.79%) megaphanerophytes, 12 (15.38%) cryptophytes, 11 (14.10%) nanophanerophytes, 5 (6.41%) hemicryptophytes and 2 (2.56%) chamaephytes. The leaf size spectra was dominated by microphyll with 31 (39.74%) species, mesophyll 17 (21.79%), nanophyll 14 (17.94%), macrophyll 6 (7.69%), leptophyll 5 (6.41%), megaphyll 2 (2.56%) and one (1.28%) was aphyllous species. Abdominal problems, jaundice, fever, wound healing, cardiac problems, eye pain, kidney pains and mouth diseases are some of diseases cured through these plants. The locals are directly dependent on the available natural resources for their subsistence. The unawareness of the collectors of medicinal plants, deforestation and overgrazing will cause endangerness and extinction of medicinal plants of the area in near future. Keywords: indigenous utilization, plant biodiversity, Malakkheil-Kotkay Valley, Shangla, Pakistan Introduction The present study area Malakkheil is a small village located on the main road towards Puran Valley in district Ethnobotany deals with the interaction between plants Shangla at 34° -31' to 33°-08' North latitude and 72° and people with particular importance on traditional -33' to 73°-01' East longitude. -

Arisaema Jacquemontii Blume (Araceae): a Review of JPP 2017; 6(6): 429-432 Received: 06-09-2017 Medicinal Uses, Phytochemistry and Pharmacology Accepted: 07-10-2017
Journal of Pharmacognosy and Phytochemistry 2017; 6(6): 429-432 E-ISSN: 2278-4136 P-ISSN: 2349-8234 Arisaema jacquemontii Blume (Araceae): A review of JPP 2017; 6(6): 429-432 Received: 06-09-2017 medicinal uses, phytochemistry and pharmacology Accepted: 07-10-2017 Rifat Roshan, Rifat Roshan, Salman Ahmed and Muhammad Mohtasheemul Hasan Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences, Abstract University of Karachi, Karachi, Arisaema jacquemontii Blume (Araceae) is a medicinally important plant and is used for the treatment of Pakistan different diseases specially in dermatological disorders. Alkaloids, phenols, terpenes, flavonoids, glycosides, tannins have been reported from this plant. Antioxidant, antifungal, antibacterial and Salman Ahmed anticancer activities are also shown by Arisaema jacquemontii. The present review is an attempt to Lecturer, Department of compile all the previous data on the basis of its medicinal uses, phytochemistry and pharmacology Pharmacognosy, Faculty of reported in the previous articles. Pharmacy and Pharmaceutical Sciences, University of Karachi, Karachi, Pakistan Keywords: Arisaema jacquemontii, medicinal uses, phytochemistry, pharmacology Muhammad Mohtasheemul 1. Introduction Hasan Arisaema jacquemontii Blume belongs to family Araceae. It is a perennial tuberous herb with Associate Professor, Department variable height from 4-28 inches. It is native to Afghanistan, China, India, Kashmir, Nepal, of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Pakistan and Sikkim. It is easily available in Himalayan forests above the sea level of 2300- [1] Sciences, University of Karachi, 4300 m and also found in upper forest and lower alpine zone in the drier areas of Himalayas Karachi, Pakistan in the range of 2400-4000 meters [2]. The species name refers to French naturalist Victor Jacquemont (1801-1832) [3]. -

Antibacterial and Antioxidant Potential of Arisaema Jacquemontii Blume from Manali, Himachal Pradesh
Bulletin of Pure and Applied Sciences. Print version ISSN 0970 4612 Vol.38 B (Botany), No.1. Jan-Jun 2019: P.23-33 Online version ISSN 2320 3196 DOI: 10.5958/2320-3196.2019.00004.1 Original Article Available online at www.bpasjournals.com Antibacterial and Antioxidant Potential of Arisaema jacquemontii Blume from Manali, Himachal Pradesh Kiran Bala1*, Jagriti Rana2, Anand Sagar3 Author’s Affiliation *Corresponding Author: 1,2,3Department of Biosciences, Himachal Kiran Bala Pradesh University, Shimla-171005, India Department of Biosciences, Himachal Pradesh University, Shimla- 171005, India E-mail: [email protected] Received on 21.01.2019, Accepted on 04.03.2019 Keywords: Abstract Arisaema jacquemonti Blume, Manali is not only a famous tourist place but also a treasure of many Antibacterial activity, traditional medicines. The antibacterial and antioxidant potential of leaves, Agar-well diffusion tubers and fruit of Arisaema jacquemonti Blume were evaluated in different method, extracts viz. acetone, chloroform, distilled water and methanol. The samples Percent inhibition of were collected from the area of Manali. Using Agar-well diffusion method bacterial growth, and different concentrations of extracts (25%, 50%, 75% and 100%), the Antioxidant potential, antibacterial activity was tested against three Gram-negative bacteria DPPH free radical (Salmonella typhi, Pseudomonas aeruginosa and Shigella dysenteriae) and two scavenging assay. Gram-positive bacteria (Lysteria monocytogenes and Staphylococcus aureus). Streptomycin (5 µg/ml) was taken as a standard antibiotic. Percent inhibition of bacterial growth was calculated for each tested extract. The tuber acetone, fruit methanol and leaf methanol extract were found to be more effective against some the tested bacteria than the standard antibiotic used. -

Analysis of the Flora
ANALYSIS OF THE FLORA The flora of the partially Himalayan district Darjeeling was not known properly. The area was surveyed by many stalwart floristic experts incliding Sir J.D. Hooker, Cambell, Griffith, Fr. Buchanan Hamilton, H. Hara, H. Ohashi, K.P. Biswas, D. G. Long, H.J. Noltie and many others. In recent years K.M. Matthew, A.P. Das and R.B. Bhujel and significantly contributing knowledge to the flora ofDarjeeling district. Even then a complete flora for the district was not available. Hills of Darjeeling and the places in Terai and Duars are well known and preferred spots for on field traing for the students of botany and forestry. R.B. Bhujel has presented the Dicotyledonous flora ofDarjeeling district in 1996 which is now in press for publication. The present work, the Monocotyledonous flora of Darjeeling will complete the angiospermic flora of this floristically extremely important district. The District of Darjeeling is a hugely populated place, though it has some quite well conserved, least interfered and even virgin places still rich in plant diversity. The most of the efficient reasons for development of such a rich floristic diversity of the region is its diversity in habitat structure, wide altitudinal ranges, unique and much variable climatic conditions, variation in adaphic and topographic conditions, high and widely distributed precipitation and the natural inter-relationship within the species. The Darjeeling region of the Eastern Himalaya has attracted a large number of tourists, botanists and naturalists from throughout the world at least for the last three centuries as it is floristically rich vegetation against the background of tallest Himalayan snow-covered peaks. -
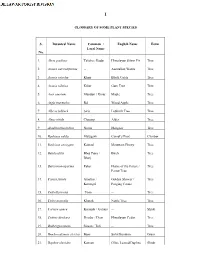
GLOSSARY of SOME PLANT SPECIES S. No. Botanical Name
I GLOSSARY OF SOME PLANT SPECIES S. Botanical Name Common / English Name Form Local Name No. 1. Abies pindrow Talisha / Badar Himalayan Silver Fir Tree 2. Acacia auriculiformis -- Australian Wattle Tree 3. Acacia catechu Khair Black Cutch Tree 4. Acacia nilotica Kikar Gum Tree Tree 5. Acer caesium Mandari / Kinar Maple Tree 6. Aegle marmelos Bel Wood Apple Tree 7. Albizia lebbeck Siris Lebback Tree Tree 8. Alnus nitida Chaamp Alder Tree 9. Azadinachta indica Neem Margosa Tree 10. Bauhinia vahlii Malugarh Camel’s Foot Climber 11. Bauhinia variegate Kakrad Mountain Ebony Tree 12. Betula utilis Bhoj Patra / Birch Tree Bharj 13. Butea monosperma Palas Flame of the Forest / Tree Parrot Tree 14. Cassia fistula Amaltas / Golden Shower / Tree Karangal Purging Cassia 15. Cedrella toona Toon -- Tree 16. Celtis australis Kharak Nettle Tree Tree 17. Carissa opaca Karonda / Garana -- Shrub 18. Cedrus deodara Deodar / Dear Himalayan Cedar Tree 19. Dalbergia sissoo Sissoo / Tali -- Tree 20. Dendrocalamus strictus Bans Solid Bamboo Grass 21. Daphne oleoides Kansan Olive Leaved Daphne Shrub II 22. Dodonaea viscose Santha Jamaica Switch Sorrel Bush 23. Emblica officinalis Amla -- Tree 24. Euphorbia royleana Thor -- Shrub 25. Fiscus bengalensis Bargad / Bod -- Tree 26. Flacourtia indica Kakoa Medagasker Plum Tree 27. Grevillea robusta -- Silver Oak Tree 28. Grewia optiva Dhamman -- Tree 29. Ilex dipyrena Khareii Himalayan Holly Tree 30. Indigofera heterantha Katai -- Shrub 31. Jacaranda mimisifolia Nili Gulmohar Mimosa Leaved Ebony Tree 32. Juglans regia Akhrot (Khod) Walnut Tree 33. Juniperus indica -- -- Tree 34. Lannea coromandelica Kembal / Jhingar -- Tree 35. Lantana camara Jadi / Panchfuli Yellow Sage Shrub 36. Lantana sillowiana Jadi / Panchfuli Weeping Lantana Shrub 37. -

Medicinal Plants of Dolpo: Amchis’ Knowledge and Conservation
Suresh K. Ghimire Yeshi Choden Lama Yildiz Aumeeruddy-Thomas in collaboration with the Amchis of Dolpo Amchis Knowledge and Conservation and Knowledge Amchis Medicinal Plants of Dolpo Medicinal Plants of Dolpo Amchis Knowledge and Conservation MedicinalAmchis Knowledge Plants and Conservation of Dolpo Yeshi Choden Lama Suresh K. Ghimire Yildiz Aumeeruddy-Thomas in collaboration with the Amchis of Dolpo People and Plants Initiative WWF Nepal Program Kathmandu, Nepal © Copyright 2001 by WWF Nepal Program Citation: LamaY.C., S.K. Ghimire and Y. Aumeeruddy-Thomas (2001). Medicinal Plants of Dolpo: Amchis’ Knowledge and Conservation. WWF Nepal Program, Kathmandu. First Edition: 1000 copies. Published in December 2001 by WWF Nepal Program PO Box: 7660, Baluwatar, Kathmandu, Nepal. Any reproduction in full or in part of this publication must mention the title and credit the above-mentioned publisher as the copyright owner. The material and the geographical designations in this report do not imply the expression of any opinion whatsoever on the part of WWF concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. Warning! Self-treatment with the herbal remedies listed in this book would be dangerous. ISBN: 99933-94-01-7 Cover photos: Courtesy: N. Budathoki, S.K. Ghimire, Y.C. Lama Photo credit inside the text: C. Basset, France (Pages: 47, 51), R.P. Chaudhary, Tribhuvan University (Pages: 40, 71, 80), S.K. Ghimire, WWF/PPI Project (Pages: 34 to 39, 41, 42, 44 to 46, 48 to 50, 52 to 66, 68 to 70, 72 to 79, 82 to 103, 105 to 111, 113 to 117, 119 to 125, 127 to 132), K.K.