Systematic Evaluation of Raninid Cuticle Microstructure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Phylogenetics of the Brachyuran Crabs (Crustacea: Decapoda): the Status of Podotremata Based on Small Subunit Nuclear Ribosomal RNA
Available online at www.sciencedirect.com Molecular Phylogenetics and Evolution 45 (2007) 576–586 www.elsevier.com/locate/ympev Phylogenetics of the brachyuran crabs (Crustacea: Decapoda): The status of Podotremata based on small subunit nuclear ribosomal RNA Shane T. Ahyong a,*, Joelle C.Y. Lai b, Deirdre Sharkey c, Donald J. Colgan c, Peter K.L. Ng b a Biodiversity and Biosecurity, National Institute of Water and Atmospheric Research, Private Bag 14901 Kilbirnie, Wellington, New Zealand b School of Biological Sciences, National University of Singapore, Kent Ridge, Singapore c Australian Museum, 6 College Street, Sydney, NSW 2010, Australia Received 26 January 2007; revised 13 March 2007; accepted 23 March 2007 Available online 13 April 2007 Abstract The true crabs, the Brachyura, are generally divided into two major groups: Eubrachyura or ‘advanced’ crabs, and Podotremata or ‘primitive’ crabs. The status of Podotremata is one of the most controversial issues in brachyuran systematics. The podotreme crabs, best recognised by the possession of gonopores on the coxae of the pereopods, have variously been regarded as mono-, para- or polyphyletic, or even as non-brachyuran. For the first time, the phylogenetic positions of the podotreme crabs were studied by cladistic analysis of small subunit nuclear ribosomal RNA sequences. Eight of 10 podotreme families were represented along with representatives of 17 eubr- achyuran families. Under both maximum parsimony and Bayesian Inference, Podotremata was found to be significantly paraphyletic, comprising three major clades: Dromiacea, Raninoida, and Cyclodorippoida. The most ‘basal’ is Dromiacea, followed by Raninoida and Cylodorippoida. Notably, Cyclodorippoida was identified as the sister group of the Eubrachyura. -

Crustacean Research 49: 237-262 (2020)
Crustacean Research 2020 Vol.49: 237–262 ©Carcinological Society of Japan. doi: 10.18353/crustacea.49.0_237 Description of a new genus for Cyrtorhina balabacensis Serène, 1971, with notes on the Cyrtorhininae (Decapoda, Brachyura, Raninidae) Peter K. L. Ng, Danièle Guinot Abstract.̶ The raninid genus Cyrtorhina Monod, 1956, is revised and restricted to Cyrtorhina granulosa Monod, 1956, from West Africa. A new genus, Flaberhina, is established for C. balabacensis Serène, 1971 from the Philippines and New Caledonia. Despite their superficial similarities, the two genera can easily be separated by the structures of the carapace, eye, antenna, pterygostome, thoracic sternum, pleon and penis. The taxonomy of the subfamily Cyrtorhininae Guinot & Ng, 2020, which con- tains both fossil and extant taxa, is also reviewed. LSID urn:lsid:zoobank.org:pub:D9C27598-A52F-4207-8C22-CDDF9E8E8FFD Key words: Raninoidea, Cyrtorhina, Flaberhina, new genus, Cyrtorhininae, systematics, review ■ Introduction until the present study. Serène & Umali (1972: 49) subsequently redescribed and figured the The raninid genus Cyrtorhina Monod, 1956, species and type specimen in greater detail. was established for one new species, Cyrtorhina balabacensis was known only from C. granulosa Monod, 1956. The holotype was the single type female of Serène (1971). a dry specimen in the Muséum national Between 2001 and 2002, however, the authors d'Histoire naturelle (MNHN), Paris, labelled as obtained three specimens of C. balabacensis “Cyrtorhina granulosa nov. sp. (A. Milne Ed- from fishermen in Balicasag Island in the cen- wards in sched.)” but the name was never pub- tral Philippines, all collected by tangle nets. lished by A. Milne Edwards. -

Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida
SHRIMPS, LOBSTERS, AND CRABS OF THE ATLANTIC COAST OF THE EASTERN UNITED STATES, MAINE TO FLORIDA AUSTIN B.WILLIAMS SMITHSONIAN INSTITUTION PRESS Washington, D.C. 1984 © 1984 Smithsonian Institution. All rights reserved. Printed in the United States Library of Congress Cataloging in Publication Data Williams, Austin B. Shrimps, lobsters, and crabs of the Atlantic coast of the Eastern United States, Maine to Florida. Rev. ed. of: Marine decapod crustaceans of the Carolinas. 1965. Bibliography: p. Includes index. Supt. of Docs, no.: SI 18:2:SL8 1. Decapoda (Crustacea)—Atlantic Coast (U.S.) 2. Crustacea—Atlantic Coast (U.S.) I. Title. QL444.M33W54 1984 595.3'840974 83-600095 ISBN 0-87474-960-3 Editor: Donald C. Fisher Contents Introduction 1 History 1 Classification 2 Zoogeographic Considerations 3 Species Accounts 5 Materials Studied 8 Measurements 8 Glossary 8 Systematic and Ecological Discussion 12 Order Decapoda , 12 Key to Suborders, Infraorders, Sections, Superfamilies and Families 13 Suborder Dendrobranchiata 17 Infraorder Penaeidea 17 Superfamily Penaeoidea 17 Family Solenoceridae 17 Genus Mesopenaeiis 18 Solenocera 19 Family Penaeidae 22 Genus Penaeus 22 Metapenaeopsis 36 Parapenaeus 37 Trachypenaeus 38 Xiphopenaeus 41 Family Sicyoniidae 42 Genus Sicyonia 43 Superfamily Sergestoidea 50 Family Sergestidae 50 Genus Acetes 50 Family Luciferidae 52 Genus Lucifer 52 Suborder Pleocyemata 54 Infraorder Stenopodidea 54 Family Stenopodidae 54 Genus Stenopus 54 Infraorder Caridea 57 Superfamily Pasiphaeoidea 57 Family Pasiphaeidae 57 Genus -

Decapod Crustacea : Raninidae
CAMPAGNES MUSORSTOM. I & II. PHILIPPINES, TOME 2 — RÉSULTATS DES CAMPAGNES MUSORSTOM. I & II. PHILIPPINES, 6 Decapod Crustacea : Raninidae Gary D. GOEKE * ABSTRACT Nine species of frog crabs of the family Raninidae were collected during the 1976 and 1980 MUSORSTOM cruises to the Philippines and the 1980 CORINDON II cruise in Makassar Strait. A proposed new genus, Lysirude (containing 3 species) is described and separated from the closely related genus Lyreidus. Five species (Raninoides hendersoni, R. personatus, Lyreidus tridentatus, L. stenops and Lysirude channeri) are represented by numerous specimens with far fewer specimens of Cosmonotus grayi, Notopoides latus, Lyreidus brevifrons and Lysirude grif- fini sp. nov. present. RÉSUMÉ Neuf espèces de Crabes de la famille des Raninidae ont été récoltées au cours des campagnes MUSORSTOM 1976 et 1980 aux Philippines et de la campagne CORINDON II dans le détroit de Macassar. Un nouveau genre Lysirude (avec trois espèces) est décrit et séparé du genre très proche Lyreidus. Cinq espèces (Raninoides hender soni, R. personatus, Lyreidus tridentatus, L. stenops et Lysirude channeri) sont représentées par de nombreux spé cimens, tandis que d'autres (Cosmonotus grayi, Notopoides latus, Lyreidus brevifrons et Lysirude griffini sp. nov.) ne comprennent qu'un nombre moins élevé d'échantillons. The Raninidae of the Philippines are a diverse group well represented in the MUSORSTOM col lections. Species of this fossorial group collected in the Philippines were most recently detailed by SERENE and UMALI (1972) and SERENE and VADON (1981). This contribution presents a systematic review of the Philippine species with an updated key to the recognized species of frog crabs known from the region. -

Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources
Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources http://www.dnr.sc.gov/marine/sertc/ Southeastern Regional Taxonomic Center Invertebrate Literature Library (updated 9 May 2012, 4056 entries) (1958-1959). Proceedings of the salt marsh conference held at the Marine Institute of the University of Georgia, Apollo Island, Georgia March 25-28, 1958. Salt Marsh Conference, The Marine Institute, University of Georgia, Sapelo Island, Georgia, Marine Institute of the University of Georgia. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Caprellidea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Gammaridea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1981). Stomatopods. FAO species identification sheets for fishery purposes. Eastern Central Atlantic; fishing areas 34,47 (in part).Canada Funds-in Trust. Ottawa, Department of Fisheries and Oceans Canada, by arrangement with the Food and Agriculture Organization of the United Nations, vols. 1-7. W. Fischer, G. Bianchi and W. B. Scott. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume II. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume III. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. -

Decapoda (Crustacea) of the Gulf of Mexico, with Comments on the Amphionidacea
•59 Decapoda (Crustacea) of the Gulf of Mexico, with Comments on the Amphionidacea Darryl L. Felder, Fernando Álvarez, Joseph W. Goy, and Rafael Lemaitre The decapod crustaceans are primarily marine in terms of abundance and diversity, although they include a variety of well- known freshwater and even some semiterrestrial forms. Some species move between marine and freshwater environments, and large populations thrive in oligohaline estuaries of the Gulf of Mexico (GMx). Yet the group also ranges in abundance onto continental shelves, slopes, and even the deepest basin floors in this and other ocean envi- ronments. Especially diverse are the decapod crustacean assemblages of tropical shallow waters, including those of seagrass beds, shell or rubble substrates, and hard sub- strates such as coral reefs. They may live burrowed within varied substrates, wander over the surfaces, or live in some Decapoda. After Faxon 1895. special association with diverse bottom features and host biota. Yet others specialize in exploiting the water column ment in the closely related order Euphausiacea, treated in a itself. Commonly known as the shrimps, hermit crabs, separate chapter of this volume, in which the overall body mole crabs, porcelain crabs, squat lobsters, mud shrimps, plan is otherwise also very shrimplike and all 8 pairs of lobsters, crayfish, and true crabs, this group encompasses thoracic legs are pretty much alike in general shape. It also a number of familiar large or commercially important differs from a peculiar arrangement in the monospecific species, though these are markedly outnumbered by small order Amphionidacea, in which an expanded, semimem- cryptic forms. branous carapace extends to totally enclose the compara- The name “deca- poda” (= 10 legs) originates from the tively small thoracic legs, but one of several features sepa- usually conspicuously differentiated posteriormost 5 pairs rating this group from decapods (Williamson 1973). -

Exceptional Preservation of Mid-Cretaceous Marine Arthropods and the Evolution of Novel Forms Via Heterochrony J
SCIENCE ADVANCES | RESEARCH ARTICLE PALEONTOLOGY Copyright © 2019 The Authors, some rights reserved; Exceptional preservation of mid-Cretaceous exclusive licensee American Association marine arthropods and the evolution of novel forms for the Advancement of Science. No claim to via heterochrony original U.S. Government J. Luque1,2,3*, R. M. Feldmann4, O. Vernygora1, C. E. Schweitzer5, C. B. Cameron6, K. A. Kerr2,7, Works. Distributed 8 9 10 1 2 under a Creative F. J. Vega , A. Duque , M. Strange , A. R. Palmer , C. Jaramillo Commons Attribution NonCommercial License 4.0 (CC BY-NC). Evolutionary origins of novel forms are often obscure because early and transitional fossils tend to be rare, poorly preserved, or lack proper phylogenetic contexts. We describe a new, exceptionally preserved enigmatic crab from the mid-Cretaceous of Colombia and the United States, whose completeness illuminates the early disparity of the group and the origins of novel forms. Its large and unprotected compound eyes, small fusiform body, and leg-like mouthparts suggest larval trait retention into adulthood via heterochronic development (pedomorphosis), while its large oar-like legs represent the earliest known adaptations in crabs for active swimming. Our phylogenetic Downloaded from analyses, including representatives of all major lineages of fossil and extant crabs, challenge conventional views of their evolution by revealing multiple convergent losses of a typical “crab-like” body plan since the Early Cretaceous. These parallel morphological transformations -
Reference List 1. Amphipacifica, Journal of Aquatic
Reference List 1. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 2. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 3. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 4. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 5. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 6. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1994. 7. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 2, 1995. 8. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1995. 9. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 2, 1995. 10. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 1, 1995. 11. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 2, 1996. 12. Amphipacifica, Journal of Aquatic Systematic Biology. Ottawa, Ontario: Amphipacifica Research Publications. Vol. 2, 1996. 13. Amphipacifica, Journal of Aquatic Systematic -

Crustacean Hosts of the Pedunculate Barnacle Genus Octolasmis in the Northern Gulf of Mexico William B
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aquila Digital Community Gulf of Mexico Science Volume 22 Article 5 Number 2 Number 2 2004 Crustacean Hosts of the Pedunculate Barnacle Genus Octolasmis in the Northern Gulf of Mexico William B. Jeffries Dickinson College Harold K. Voris Field Museum of Natural History DOI: 10.18785/goms.2202.05 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Jeffries, W. B. and H. K. Voris. 2004. Crustacean Hosts of the Pedunculate Barnacle Genus Octolasmis in the Northern Gulf of Mexico. Gulf of Mexico Science 22 (2). Retrieved from https://aquila.usm.edu/goms/vol22/iss2/5 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Jeffries and Voris: Crustacean Hosts of the Pedunculate Barnacle Genus Octolasmis in Gulf of Mexico Science, 2004(2), pp. 173--188 Crustacean Hosts of the Pedunculate Barnacle Genus Octolasmis in the Northern Gulf of Mexico WILLIAM B. JEFFRIES AND HAROLD K. VoRis A survey of live and preserved crustaceans from the northern portion of the Gulf of Mexico was conducted to investigate the colonization habits of the bar nacle genus Octolasmis. In all, three crustacean orders (Decapoda, lsopoda, and Stomatopoda) comprising 43 families, 78 genera, and 122 species were surveyed. Octolasmis barnacles were observed to infest 14 families, 20 genera, and 27 species of the orders Decapoda and Isopoda. -

Decapod Crustaceans from the Paleocene of Central Texas, USA
Revista Mexicana de Ciencias Geológicas,Decapod v. crustaceans 26, núm. 3, from2009, the p. 745-763Paleocene of Central Texas, USA 745 Decapod crustaceans from the Paleocene of Central Texas, USA Adam Armstrong1, Torrey Nyborg2, Gale A. Bishop3, Àlex Ossó-Morales4, and Francisco J. Vega5,* 1 306 Hilltop Road, Keene, TX 76059, U.S.A. 2 Department of Earth and Biological Sciences, Loma Linda University, Loma Linda, California 92350, U.S.A. 3 Department of Geology and Geography, Georgia Southern University, Statesboro, GA 30460, U.S.A. 4 Josep Vicenç Foix, 12-H, 1er-1ª 43007 Tarragona, Catalonia, Spain. 5 Instituto de Geología, Universidad Nacional Autónoma de México, Ciudad Universitaria, Coyoacán, México DF 04510, Mexico. * [email protected] ABSTRACT Fourteen species of decapods crustaceans are described from a single locality near Mexia, Texas, where Paleocene sediments of the Mexia Clay Member of the Wills Point Formation crop out. The species are represented by Hoploparia sp., Linuparus wilcoxensis Rathbun, 1935, an unnamed paguroid, Kierionopsis nodosa Davidson, 1966, Pithonoton cardwelli new species, Caloxanthus sp., Macroacaena johnsoni (Rathbun, 1935), new combination, Raninoides bournei (Rathbun, 1928), R. treldenaesensis (Collins and Jakobsen, 2003), Prehepatus sp., Tehuacana americana (Rathbun, 1935), new combination, Costacopluma texana new species, Paraverrucoides alabamensis (Rathbun, 1935) and Viapinnixa mexiaensis new species. Morphological details unknown for previously described species are included. New systematic placements are offered based on recent research. Intraspecifi c morphological variation is documented for the goneplacoid crab Tehuacana americana. Costacopluma texana is the second Paleogene species of that genus in southeastern USA, and because of its abundance represents the most completely described species for the genus. -
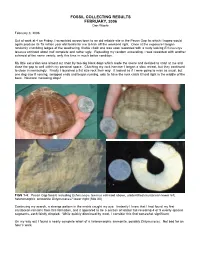
FOSSIL COLLECTING RESULTS FEBRUARY, 2006 Dan Woehr
FOSSIL COLLECTING RESULTS FEBRUARY, 2006 Dan Woehr February 3, 2006 Out of work at 4 on Friday, I scrambled across town to an old reliable site in the Pecan Gap fm which I hoped would again produce its 75 million year old fossils for me to kick off the weekend right. Once at the exposure I began randomly crumbling ledges of the weathering, friable chalk and was soon rewarded with a rusty looking Echinocorys texanus echinoid about half complete and rather ugly. Repeating my random excavating, I was rewarded with another echinoid of the same variety, only this time in much better condition. My little excursion was almost cut short by two big black dogs which made the scene and decided to snarl at me and close the gap to well within my personal space. Clutching my rock hammer I began a slow retreat, but they continued to close in menacingly. Finally I launched a fist size rock their way. It looked as if I were going to miss as usual, but one dog saw it coming, swapped ends and began running, only to have the rock catch it hard right in the middle of the back. No more menacing dogs! FIGS 1-4: Pecan Gap fossils including Echinocorys texanus echinoid above, unidentified crustacean lower left, heteromorphic ammonite Didymoceras? lower right (Site 20) Continuing my search, a strange pattern in the matrix caught my eye. Instantly I knew that I had found my first crustacean remains from this formation, and it appeared to be a section of lobster tail revealing 4 or 5 evenly spaced segments, each faintly dimpled.