University of Southampton Research Repository Eprints Soton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
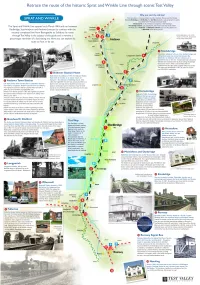
Sprat and Winkle Line Leaflet
k u . v o g . y e l l a v t s e t @ e v a e l g d t c a t n o c e s a e l P . l i c n u o C h g u o r o B y e l l a V t s e T t a t n e m p o l e v e D c i m o n o c E n i g n i k r o w n o s n i b o R e l l e h c i M y b r e h t e g o t t u p s a w l a i r e t a m e h T . n o i t a m r o f n I g n i d i v o r p r o f l l e s d n i L . D r M d n a w a h s l a W . I r M , n o t s A H . J r M , s h p a r g o t o h p g n i d i v o r p r o f y e l r e s s a C . R r M , l l e m m a G . C r M , e w o c n e l B . R r M , e n r o H . M r M , e l y o H . R r M : t e l f a e l e l k n i W d n a t a r p S e h t s d r a w o t n o i t a m r o f n i d n a s o t o h p g n i t u b i r t n o c r o f g n i w o l l o f e h t k n a h t o t e k i l d l u o w y e l l a V t s e T s t n e m e g d e l w o n k c A . -

T4 Bus Time Schedule & Line Route
T4 bus time schedule & line map T4 Totton - Cadnam - Totton View In Website Mode The T4 bus line (Totton - Cadnam - Totton) has 2 routes. For regular weekdays, their operation hours are: (1) Totton: 10:00 AM - 12:00 PM (2) West Totton: 2:00 PM Use the Moovit App to ƒnd the closest T4 bus station near you and ƒnd out when is the next T4 bus arriving. Direction: Totton T4 bus Time Schedule 56 stops Totton Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday Not Operational St Theresas Church, Totton Tuesday 10:00 AM - 12:00 PM Asda, Totton Ringwood Road, Totton And Eling Civil Parish Wednesday Not Operational Bagber Road, Totton Thursday 10:00 AM - 12:00 PM Friday Not Operational Forest Park School, Totton Southern Gardens, Totton And Eling Civil Parish Saturday Not Operational Calmore Corner, West Totton Graddidge Way, West Totton Galsworthy Road, Totton And Eling Civil Parish T4 bus Info Direction: Totton Hazel Farm Road, West Totton Stops: 56 Redwood Gardens, Totton And Eling Civil Parish Trip Duration: 50 min Line Summary: St Theresas Church, Totton, Asda, Crabbs Way, West Totton Totton, Bagber Road, Totton, Forest Park School, Totton, Calmore Corner, West Totton, Graddidge Way, Goodies, West Totton West Totton, Hazel Farm Road, West Totton, Crabbs Way, West Totton, Goodies, West Totton, Morrisons, Morrisons, West Totton West Totton, Stonechat Drive, West Totton, Michigan Skylark Walk, Totton And Eling Civil Parish Way, Calmore, Amey Gardens, Calmore, The Drove, Calmore, Farm Close, Calmore, Woodhaven & Stonechat Drive, -

A CRITICAL EVALUATION of the LOWER-MIDDLE PALAEOLITHIC ARCHAEOLOGICAL RECORD of the CHALK UPLANDS of NORTHWEST EUROPE Lesley
A CRITICAL EVALUATION OF THE LOWER-MIDDLE PALAEOLITHIC ARCHAEOLOGICAL RECORD OF THE CHALK UPLANDS OF NORTHWEST EUROPE The Chilterns, Pegsdon, Bedfordshire (photograph L. Blundell) Lesley Blundell UCL Thesis submitted for the degree of PhD September 2019 2 I, Lesley Blundell, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed: 3 4 Abstract Our understanding of early human behaviour has always been and continues to be predicated on an archaeological record unevenly distributed in space and time. More than 80% of British Lower-Middle Palaeolithic findspots were discovered during the late 19th/early 20th centuries, the majority from lowland fluvial contexts. Within the British planning process and some academic research, the resultant findspot distributions are taken at face value, with insufficient consideration of possible bias resulting from variables operating on their creation. This leads to areas of landscape outside the river valleys being considered to have only limited archaeological potential. This thesis was conceived as an attempt to analyse the findspot data of the Lower-Middle Palaeolithic record of the Chalk uplands of southeast Britain and northern France within a framework complex enough to allow bias in the formation of findspot distribution patterns and artefact preservation/discovery opportunities to be identified and scrutinised more closely. Taking a dynamic, landscape = record approach, this research explores the potential influence of geomorphology, 19th/early 20th century industrialisation and antiquarian collecting on the creation of the Lower- Middle Palaeolithic record through the opportunities created for artefact preservation and release. -
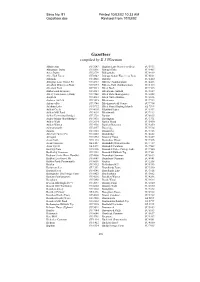
Gazetteer.Doc Revised from 10/03/02
Save No. 91 Printed 10/03/02 10:33 AM Gazetteer.doc Revised From 10/03/02 Gazetteer compiled by E J Wiseman Abbots Ann SU 3243 Bighton Lane Watercress Beds SU 5933 Abbotstone Down SU 5836 Bishop's Dyke SU 3405 Acres Down SU 2709 Bishopstoke SU 4619 Alice Holt Forest SU 8042 Bishops Sutton Watercress Beds SU 6031 Allbrook SU 4521 Bisterne SU 1400 Allington Lane Gravel Pit SU 4717 Bitterne (Southampton) SU 4413 Alresford Watercress Beds SU 5833 Bitterne Park (Southampton) SU 4414 Alresford Pond SU 5933 Black Bush SU 2515 Amberwood Inclosure SU 2013 Blackbushe Airfield SU 8059 Amery Farm Estate (Alton) SU 7240 Black Dam (Basingstoke) SU 6552 Ampfield SU 4023 Black Gutter Bottom SU 2016 Andover Airfield SU 3245 Blackmoor SU 7733 Anton valley SU 3740 Blackmoor Golf Course SU 7734 Arlebury Lake SU 5732 Black Point (Hayling Island) SZ 7599 Ashlett Creek SU 4603 Blashford Lakes SU 1507 Ashlett Mill Pond SU 4603 Blendworth SU 7113 Ashley Farm (Stockbridge) SU 3730 Bordon SU 8035 Ashley Manor (Stockbridge) SU 3830 Bossington SU 3331 Ashley Walk SU 2014 Botley Wood SU 5410 Ashley Warren SU 4956 Bourley Reservoir SU 8250 Ashmansworth SU 4157 Boveridge SU 0714 Ashurst SU 3310 Braishfield SU 3725 Ash Vale Gravel Pit SU 8853 Brambridge SU 4622 Avington SU 5332 Bramley Camp SU 6559 Avon Castle SU 1303 Bramshaw Wood SU 2516 Avon Causeway SZ 1497 Bramshill (Warren Heath) SU 7759 Avon Tyrrell SZ 1499 Bramshill Common SU 7562 Backley Plain SU 2106 Bramshill Police College Lake SU 7560 Baddesley Common SU 3921 Bramshill Rubbish Tip SU 7561 Badnam Creek (River -

36 Bus Time Schedule & Line Route
36 bus time schedule & line map 36 Lockerley View In Website Mode The 36 bus line (Lockerley) has 2 routes. For regular weekdays, their operation hours are: (1) Lockerley: 12:51 PM (2) Romsey: 9:26 AM - 1:26 PM Use the Moovit App to ƒnd the closest 36 bus station near you and ƒnd out when is the next 36 bus arriving. Direction: Lockerley 36 bus Time Schedule 23 stops Lockerley Route Timetable: VIEW LINE SCHEDULE Sunday Not Operational Monday Not Operational Bus Station, Romsey Tuesday 12:51 PM Council O∆ces, Romsey Church Place, Romsey Wednesday Not Operational Malthouse Close, Romsey Thursday 12:51 PM Malthouse Close, Romsey Friday Not Operational Priestlands, Romsey Saturday Not Operational Greatbridge Road, Romsey Fishlake Meadows, Romsey Dukes Head, Belbins 36 bus Info Direction: Lockerley Timsbury Institute, Timsbury Stops: 23 Trip Duration: 34 min Recreation Ground, Michelmersh Line Summary: Bus Station, Romsey, Council O∆ces, Romsey, Malthouse Close, Romsey, Priestlands, Mannyngham Way, Michelmersh Romsey, Fishlake Meadows, Romsey, Dukes Head, Belbins, Timsbury Institute, Timsbury, Recreation Hill View Road, Michelmersh Ground, Michelmersh, Mannyngham Way, Michelmersh, Hill View Road, Michelmersh, Brickworks, Michelmersh, Bear And Ragged Staff, Brickworks, Michelmersh Kimbridge, Mottisfont Abbey, Mottisfont, Bengers Lane, Mottisfont, Village Hall, Mottisfont, Russell Bear And Ragged Staff, Kimbridge Drive, Dunbridge, Awbridge School, Kents Oak, Church Lane, Awbridge, Wood Farm, Kents Oak, Mottisfont Abbey, Mottisfont Newtown, Doctor's -

Beaulieu Bramshaw Brockenhurst Copythorne
New Forest National Park Authority Date: 12/05/2021 Parish List Trees Works Applications Decided Between 05/05/21 and 11/05/21 BEAULIEU Application No: R14/15/21/0231 Address: SPEARBED COPSE, DOCK LANE, BEAULIEU, BROCKENHURST, SO42 7YJ Case Officer: Nik Gruber Decision Date: 05/05/2021 Decision: Exempt Works BRAMSHAW Application No: CONS/21/0189 Address: POPES COTTAGE, LYNDHURST ROAD, BROOK, LYNDHURST, SO43 7HE Case Officer: James Palmer Decision Date: 11/05/2021 Decision: Raise No Objections BROCKENHURST Application No: TPO/21/0188 Address: THE GRACES, ARMSTRONG ROAD, BROCKENHURST, SO42 7TA Case Officer: Nik Gruber Tree Ref Proposed Works Reason for Work ALL T23 Fell 1 x Red Oak leaving a 4m habitat Degraded stem base with Armillaria ssp monolith. present, Decision Date: 06/05/2021 Decision: Grant COPYTHORNE Application No: CONS/21/0182 Address: THE COTTAGE, NEWBRIDGE, CADNAM, SOUTHAMPTON, SO40 2NW Case Officer: James Palmer Decision Date: 06/05/2021 Decision: Raise No Objections HALE Application No: CONS/21/0181 Address: ARWOOD, HALE PURLIEU, HALE, FORDINGBRIDGE, SP6 2NN Case Officer: James Palmer Decision Date: 06/05/2021 Decision: Raise No Objections HYDE Application No: R14/15/21/0235 Address: 2 THE PADDOCK, FROGHAM, FORDINGBRIDGE, SP6 2HZ Case Officer: Nik Gruber Decision Date: 07/05/2021 Decision: Exempt Works LYNDHURST Q:\Plantech\Reports\TP\TWAPPS\Decided Between Dates(Tree Works).rpt Page 1 of 2 New Forest National Park Authority Date: 12/05/2021 Parish List Trees Works Applications Decided Between 05/05/21 and 11/05/21 Application -

M+W Sites List (HF000007092018)
Hampshire County Council Site Code Site Name Grid Ref Operator / Agent Site Description Site Status Site Narrative (* = Safeguarded), (†=Chargeable site) Basingstoke and Deane Borough Council BA009 Newnham Common 470336 Hampshire County Council Landfill (restored) Site completed Restored non-inert landfill, closed in 1986 but subject to leachate monitoring not monitored (closed site, low priority) 153471 BA017† Apple Dell 451004 Portals Landfill (inert) Lapsed permission Dormant; deposit of non-toxic cellulose waste from paper making processes for a period of ten years (BDB46323) (Agriculture - 2009) Permission expired, no monitoring. Overton 148345 BA018* Wade Road 465127 Basingstoke Skip Hire, Hampshire County Council, Waste Processing, HWRC Active Waste transfer, including construction, demolition, industrial, household and clinical waste and household waste recycling centre; extension and improvement of household waste recycling Basingstoke 153579 Veolia Environmental Services (UK) Plc centre (BDB/60584); erection of waste recycling building (BDB/61845); erection of extension to existing waste recycling building (BDB/64564); extension and improvement of household waste recycling centre (BDB/69806) granted 12.2008 - 2 monitoring visits per year. BA019* Chineham Energy Recovery Facility 467222 Veolia Environmental Services (UK) Plc Waste Processing (energy Active Energy recovery incinerator (BDB044300) commissioned in autumn 2002 with handover in January 2003; the incinerator has the capacity to process at least 90,000 tonnes a year, -

The Middleton Estate
WELCOME TO THE MIDDLETON ESTATE Dear Angler, Welcome to the Middleton Estate! By now I hope you are settled and are relaxing with a cup of coffee. Here is a summary of the fishing and what to expect; have a lovely day. THE RIVER TEST The River Test has a total length of 40 miles and flows through the Hampshire downlands from its source near Overton, 6 miles to the west of Basingstoke, to the sea at the head of Southampton Water. The river rises in the village of Ashe, and flows west through the villages of Overton, Laverstoke, and the town of Whitchurch, before joining with the Bourne Rivulet at Testbourne and turning into a more southerly direction. It then flows through the villages of Longparish and Middleton to Wherwell and Chilbolton, where the Rivers Dever and Anton contribute to the flow. From Chilbolton the river flows through the villages of Leckford, Longstock, Stockbridge and Houghton to Mottisfont and Kimbridge, where the River Dun joins the flow. From here the village of Timsbury is passed, then through the grounds of Roke Manor before reaching the town of Romsey. On the western edge of Romsey, Sadler's Mill, an 18th Century watermill, sits astride the River Test. South of Romsey, the river flows past the country house of Broadlands, past Nursling that was once the site of a Roman bridge, and between Totton and Redbridge. Here the river is joined by the River Blackwater and soon becomes tidal, widening out into a considerable estuary that is lined on its northern bank by the container terminals and quays of the Port of Southampton. -

Sites of Importance for Nature Conservation Sincs Hampshire.Pdf
Sites of Importance for Nature Conservation (SINCs) within Hampshire © Hampshire Biodiversity Information Centre No part of this documentHBIC may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recoding or otherwise without the prior permission of the Hampshire Biodiversity Information Centre Central Grid SINC Ref District SINC Name Ref. SINC Criteria Area (ha) BD0001 Basingstoke & Deane Straits Copse, St. Mary Bourne SU38905040 1A 2.14 BD0002 Basingstoke & Deane Lee's Wood SU39005080 1A 1.99 BD0003 Basingstoke & Deane Great Wallop Hill Copse SU39005200 1A/1B 21.07 BD0004 Basingstoke & Deane Hackwood Copse SU39504950 1A 11.74 BD0005 Basingstoke & Deane Stokehill Farm Down SU39605130 2A 4.02 BD0006 Basingstoke & Deane Juniper Rough SU39605289 2D 1.16 BD0007 Basingstoke & Deane Leafy Grove Copse SU39685080 1A 1.83 BD0008 Basingstoke & Deane Trinley Wood SU39804900 1A 6.58 BD0009 Basingstoke & Deane East Woodhay Down SU39806040 2A 29.57 BD0010 Basingstoke & Deane Ten Acre Brow (East) SU39965580 1A 0.55 BD0011 Basingstoke & Deane Berries Copse SU40106240 1A 2.93 BD0012 Basingstoke & Deane Sidley Wood North SU40305590 1A 3.63 BD0013 Basingstoke & Deane The Oaks Grassland SU40405920 2A 1.12 BD0014 Basingstoke & Deane Sidley Wood South SU40505520 1B 1.87 BD0015 Basingstoke & Deane West Of Codley Copse SU40505680 2D/6A 0.68 BD0016 Basingstoke & Deane Hitchen Copse SU40505850 1A 13.91 BD0017 Basingstoke & Deane Pilot Hill: Field To The South-East SU40505900 2A/6A 4.62 -

Report Re A336 7.5 Tonne Weight Limit Proposal
COPYTHORNE PARISH COUNCIL MEETING – 13th APRIL 2021 ITEM 10 – A336 (Southampton Road through Cadnam & Bartley and Ringwood Road through Netley Marsh) – Proposal to HCC Highways for a 7.5 tonne Weight Limit Background At the March 9th 2021 Council meeting, a resident of Netley Marsh spoke in the public session about a proposal being presented to Netley Marsh Parish Council seeking their support for a 7.5 tonne weight limit on the A336 through Netley Marsh. Given that the A336 continues westwards from Netley Marsh through Bartley and Cadnam, similar support was sought from Copythorne Parish Council. Current position Netley Marsh Parish Council has confirmed it’s support and we have been provided with a copy of the document giving full details of the proposal that will be submitted to HCC Highways (Appendix 1) to this report. (Note:- In the first paragraph of Section 2 (Introduction) of the document reference is made to the proposal being “supported by Netley Marsh Parish and Copythorne Parish Councils on behalf of the residents of the impacted villages.” The support of Copythorne Parish Council is being requested and has not yet been agreed. Key Points to consider i. Such a weight limit would greatly reduce the level of larger vehicles using the Southampton Road through the Parish. ii. A 7.5 tonne weight limit is already in place from the A336 roundabout with the A326 (“Goodies”) through to the centre of Totton. iii. The limit would not apply to vehicles requiring access to businesses etc. along the route, such as to the saw mill at Bartley, the garden centre at Cadnam, school buses, etc. -

Esconet Text Retrieval System
EscoNet Text Retrieval System TEST VALLEY BOROUGH COUNCIL - PLANNING SERVICES WEEKLY LIST OF PLANNING APPLICATIONS AND NOTIFICATIONS : NO. 40 Week Ending: Friday, 1 October 2004 Comments on any of these matters should be forwarded IN WRITING to arrive before the expiry date shown in the last column For the Northern Area (TVN) to: For the Southern Area (TVS) to Head of Planning Head of Planning Council Offices 'Beech Hurst' Duttons Road Weyhill Road ROMSEY SO51 8XG ANDOVER SPIO 3AJ In accordance with the provisions of the Local Government (Access to Information Act) 1985, any representations received may be open to public inspection PLANNING APPLICATIONS APPLICATION NO. / PROPOSAL LOCATION APPLICANT CASE OFFICER / REGISTRATION DATE PUBLICITY EXPIRY DATE http://www.minutes.org.uk/cgi-bin/cgi003.exe?Y,,,00000000000000100000...,,01.10.04,14223067,14295901,1,011004.HTM,1,1,1,P,67798058,0,00,00,N (1 of 6) [06/06/2013 17:03:40] EscoNet Text Retrieval System TVN.09223 Erection of rear conservatory 3 Elder Crescent, ABBOTTS ANN Mr S Grainger Maggie Francis 28.09.2004 Ms K M Auton 26.10.2004 TVN.09242 Erection of conservatory to rear 1 Stone Close, Andover Mr And Mrs Woodward Paul Goodman 28.09.2004 MILLWAY 26.10.2004 TVN.08963/1 Erection of two storey extension to 16 The Avenue, Andover Mr G Kitchen William Josey 28.09.2004 provide conservatory and garage MILLWAY Miss J Sellwood 26.10.2004 with bedroom and en-suite over TPO.00832/11 Gradual removal of whole conifer Tillers, Barncroft, APPLESHAW Mr And Mrs C & S Jones Dermot Cox 27.09.2004 hedge over a period of five years 26.10.2004 TVN.LB.00084/6 Installation of architectural lighting The Guildhall, High Street, Urban Projects Limited William Josey 27.09.2004 scheme Andover ST MARY'S 29.10.2004 TRE.CA.00587/97 Cotoneaster tree - Shorten back Fleur De Lys, AMPORT Andrew Douglas 27.09.2004 some branches on house side by one 26.10.2004 third. -

RESULTS 1 Awards List 2 Estate Results 3 Final Rankings 4 Final
RESULTS 1 Awards list 2 Estate results 3 Final rankings 4 Final teams cores 5 Estate scores 6 Fly results For more information or to enter for 2016 (Friday April 22nd) contact Simon Cooper Fishing Breaks The Mill Heathman Street Nether Wallop Hampshire SO20 8EW 01264 781988 [email protected] www.fishingbreaks.co.uk/onefly 2015 River Test One Fly Roll of Honour Winning Team Award Points 1st Stream Dreamers John Graham/Alastair Robjent/Marcus Janssen 2525 2nd TheWellowWaderWallowers Darren Dodd/Wes Boyd/Chris Banks 2240 3rd Minley Manor Moustaches Grant Harrower/Iain Shone/Paul Quilliam 1950 A trophy for each team member. Top Angler & Guide Marcus Janssen & Brian Raw 1st Pheasant Tail Hare's Ear with tungsten bead and red butt 1370 2nd Grant Harrower & Charlie Patrick 1250 3rd Brian Reid & Dave Barnes 1155 Angler: Trophy Guide: Trophy & cheque for £500. Angler & Guide Section Winners A Charles Jardine & John Colley @ Benham Estate B Marcus Janssen & Brian Raw @ Wherwell Priory C Chris Banks & Bob Preston @ Avon Springs Anglers: Trophy Guides: Trophy & cheque for £100. Top Estate 1st Compton Chamberlayne 2nd Whitchurch Fulling Mill 3rd Benham Estate A trophy. Big Fish Award Jan Grimstone/Malcolm Price @ Wherwell Priory and Alastair Robjent/John Slader @ Orvis Kimbridge 24" £750 raised for the Alex Lewis Trust Duffers Delight Award for the lowest scoring team The Dream Team A trophy for each team member. Top dry fly (highest score) Alastair Robjent at Orvis @ Kimbridge with Robjents Daddy A trophy. RTOF 2015 final scoresheets - ratified 2015 Estate Roll of Honour Top Estate by total score/no.