IEC 60601-1 Medical Electrical Equipment Part 1: General Requirements for Basic Safety and Essential Performance Report Reference No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

IEC-International Electrotechnical Commission
Standards Manager Web Standards List IEC-International Electrotechnical Commission Id Number Title Year Organization Page 1 60034-2-3 Rotating electrical machines _ Part 2-3: Specific test methods for determining losses and efficiency of converter-fed AC 2020 IEC motors - Edition 1.0 2 60034-3 Rotating electrical machines _ Part 3: Specific requirements for synchronous generators driven by steam turbines or 2020 IEC combustion gas turbines and for synchronous compensators - Edition 7.0 3 60034-5 Rotating electrical machines _ Part 5: Degrees of protection provided by the integral design of rotating electrical machines 2020 IEC (IP code) _ Classification - Edition 5.0 4 60034-7 Rotating electrical machines _ Part 7: Classification of types of construction, mounting arrangements and terminal box 2020 IEC position (IM Code) - Edition 3.0 5 60034-11 Rotating electrical machines _ Part 11: Thermal protection - Edition 3.0 2020 IEC 6 60034-18-42 Rotating electrical machines _ Part 18-42: Partial discharge resistant electrical insulation systems (Type II) used in rotating 2020 IEC electrical machines fed from voltage converters _ Qualification tests - Edition 1.1; Consolidated Reprint 7 60045-1 Steam turbines _ Part 1: Specifications - Edition 2.0 2020 IEC 8 60050-113 NULL 2020 IEC AMD 2 9 60050-113 AMENDMENT 3 International Electrotechnical Vocabulary (IEV) _ Part 113: Physics for electrotechnology - Edition 1.0 2020 IEC AMD 3 10 60050-151 AMENDMENT 4 International Electrotechnical Vocabulary (IEV) _ Part 151: Electrical and magnetic devices -

Nr. Standard Reference Title 1 IEC 60317-4:2015
Nr. Standard reference Title Specifications for particular types of winding wires - Part 4: Solderable 1 IEC 60317-4:2015 polyurethane enamelled round copper wire, class 130 Appliance couplers for household and similar general purposes - Part 1: 2 IEC 60320-1:2015 General requirements Amendment 1 - Low-voltage electrical installations – Part 4-44: Protection IEC 60364-4- 3 for safety – Protection against voltage disturbances and electromagnetic 44:2007/AMD1:2015 PRV disturbances IEC 60364-5- Amendment 2 - Electrical installations of buildings – Part 5-53: Selection 4 53:2001/AMD2:2015 PRV and erection of electrical equipment – Isolation, switching and control Corrigendum 1 - High-voltage swithgear and controlgear - Part 200: AC IEC 62271- 5 metal-enclosed switchgear and controlgear for rated voltages above 1 kV 200:2011/COR1:2015 and up to and including 52 kV IEC/IEEE 62271-37- High-voltage switchgear and controlgear – Part 37-013: Alternating-current 6 013:2015 PRV generator circuit-breakers Photobiological safety of lamps and lamp systems - Part 5: Image 7 IEC 62471-5:2015 projectors LEDsi lamps for general lighting services with supply voltages not 8 IEC 62838:2015 PRV exceeding 50 V a.c. r.m.s. or 120 V ripple free d.c. – Safety specifications IEC Corrigendum 1 - Amendment 1 - Self-ballasted LED-lamps for general 9 62560:2011/AMD1:2015/CO lighting services by voltages >50 V - Safety specifications R1:2015 Electrical installations for lighting and beaconing of aerodromes – Safety 10 IEC 62870:2015 PRV secondary circuits in series circuits -

SAV4118 Layout
PUBLISHED WEEKLY BY THE AMERICAN NATIONAL STANDARDS INSTITUTE 25 West 43rd Street, NY, NY 10036 VOL. 41, #18 April 30, 2010 Contents American National Standards Call for Comment on Standards Proposals ................................................. 2 Call for Comment Contact Information ........................................................ 17 Call for Members (ANS Consensus Bodies)................................................ 19 Final Actions .................................................................................................. 23 Project Initiation Notification System (PINS)............................................... 25 International Standards ISO Draft Standards....................................................................................... 31 ISO Newly Published Standards................................................................... 32 Proposed Foreign Government Regulations................................................. 33 Information Concerning .................................................................................. 34 American National Standards Call for comment on proposals listed This section solicits public comments on proposed draft new Ordering Instructions for "Call-for-Comment" Listings American National Standards, including the national adoption of 1. Order from the organization indicated for the specific ISO and IEC standards as American National Standards, and on proposal. proposals to revise, reaffirm or withdraw approval of existing American National Standards. A draft -

Zephyr Overview 091520.Pdf
Zephyr Project: Unlocking Innovation with an Open Source RTOS Kate Stewart @_kate_stewart [email protected] Copyright © 2020 The Linux Foundation. Made avaiilable under Attribution-ShareAlike 4.0 International Zephyr Project Open Source, RTOS, Connected, Embedded • Open source real time operating system Fits where Linux is too big • Vibrant Community participation • Built with safety and security in mind • Cross-architecture with broad SoC and Zephyr OS development board support. 3rd Party Libraries • Vendor Neutral governance Application Services • Permissively licensed - Apache 2.0 OS Services • Complete, fully integrated, highly Kernel configurable, modular for flexibility HAL • Product development ready using LTS includes security updates • Certification ready with Auditable Products Running Zephyr Today Grush Gaming hereO Proglove Rigado IoT Gateway Distancer Toothbrush Smartwatch Ellcie-Healthy Smart Intellinium Safety Anicare Reindeer GNARBOX 2.0 SSD Adero Tracking Devices Sentrius Connected Eyewear Shoes Tracker GEPS Point Home Alarm RUUVI Node HereO Core Box Safety Pod Zephyr Supported Hardware Architectures Cortex-M, Cortex-R & Cortex-A X86 & x86_64 32 & 64 bit Xtensa Coming soon: Native Execution on a POSIX-compliant OS • Build Zephyr as native Linux application • Enable large scale simulation of network or Bluetooth tests without involving HW • Improve test coverage of application layers • Use any native tools available for debugging and profiling • Develop GUI applications entirely on the desktop • Optionally connect -
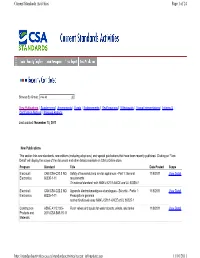
Page 1 of 24 Current Standards Activities 11/10/2011 Http
Current Standards Activities Page 1 of 24 Browse By Group: View All 6 New Publications | Supplements | Amendments | Errata | Endorsements | Reaffirmations | Withdrawals | Formal Interpretations | Informs & Certification Notices | Previous Reports Last updated: November 10, 2011 New Publications This section lists new standards, new editions (including adoptions), and special publications that have been recently published. Clicking on "View Detail" will display the scope of the document and other details available on CSA’s Online store. Program Standard Title Date Posted Scope Electrical / CAN/CSA-C22.2 NO. Safety of household and similar appliances - Part 1: General 11/8/2011 View Detail Electronics 60335-1-11 requirements Tri-national standard, with NMX-J-521/1-ANCE and UL 60335-1 Electrical / CAN/CSA-C22.2 NO. Appareils électrodomestiques et analogues - Sécurité - Partie 1: 11/8/2011 View Detail Electronics 60335-1-11 Prescriptions generals norme trinationale avec NMX-J-521/1-ANCE et UL 60335-1 Construction ASME A112.19.5- Flush valves and spuds for water closets, urinals, and tanks 11/8/2011 View Detail Products and 2011/CSA-B45.15-11 Materials http://standardsactivities.csa.ca/standardsactivities/recent_infoupdate.asp 11/10/2011 Current Standards Activities Page 2 of 24 Construction ASSE 1016- Performance requirements for automatic compensating valves for 11/8/2011 View Detail Products and 2011/ASME individual showers and tub/shower combinations Materials A112.1016-2011/CSA B125.16-11 Energy C823-11 Rendement des appareils de traitement -

International Standard Iec 60601-1
INTERNATIONAL IEC STANDARD 60601-1 Third edition 2005-12 Medical electrical equipment – Part 1: General requirements for basic safety and essential performance This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French- language pages. Reference number IEC 60601-1:2005(E) Publication numbering As from 1 January 1997 all IEC publications are issued with a designation in the 60000 series. For example, IEC 34-1 is now referred to as IEC 60034-1. Consolidated editions The IEC is now publishing consolidated versions of its publications. For example, edition numbers 1.0, 1.1 and 1.2 refer, respectively, to the base publication, the base publication incorporating amendment 1 and the base publication incorporating amendments 1 and 2. Further information on IEC publications The technical content of IEC publications is kept under constant review by the IEC, thus ensuring that the content reflects current technology. Information relating to this publication, including its validity, is available in the IEC Catalogue of publications (see below) in addition to new editions, amendments and corrigenda. Information on the subjects under consideration and work in progress undertaken by the technical committee which has prepared this publication, as well as the list of publications issued, is also available from the following: • IEC Web Site (www.iec.ch) • Catalogue of IEC publications The on-line catalogue on the IEC web site (www.iec.ch/searchpub) enables you to search by a variety of criteria including text searches, technical committees and date of publication. -

VP IEC Júl 2012
NÁVRHY NORIEM IEC PREDLOŽENÝCH NA VEREJNÉ PREROKOVANIE za obdobie od 1. júla 2012 do 31. júla 2012 Documents Title Closing Date 1/2210A/FDIS Amendment 1 to IEC 60050-151: International Electrotechnical Vocabulary - Part 151: Electrical and magnetic devices (Proposed horizontal 2012-10-12 standard) 3/1108/CD IEC 61082/Ed.3: PREPARATION OF DOCUMENTS USED IN ELECTROTECHNOLOGY, Part 1: Rules 2012-10-12 7/622/CD IEC 62774/Ed1: Conductors for overhead lines - Coated metallic wire for concentric lay stranded conductors 2012-10-12 9/1696A/CDV IEC 62724 Ed.1: Railway applications - Fixed installations - Electric traction - Insulating synthetic rope assemblies for support of overhead 2012-12-07 contact lines 9/1711/FDIS IEC 60571 Ed.3: Railway applications - Electronic equipment used on rolling stock 2012-09-21 9/1712/CD IEC 62290-1 Ed.2: Railway applications - Urban guided transport management and command/control systems - Part 1: System principles 2012-10-26 and fundamental concepts 9/1713/CD IEC 62290-2 Ed.2: Railway applications - Urban guided transport management and command/control systems - Part 2: Functional 2012-10-26 requirements specification 10/890/CD IEC 60839 Ed.3: Specifications for unused silicone insulating liquids for electrotechnical purposes 2012-10-19 17A/1018/CDV Amendment 1 to IEC 62271-109 Ed2: High-voltage switchgear and controlgear - Part 109: Alternating current series capacitor by-pass 2012-12-07 switches 17B/1783/CDV IEC 60947-6-1 am1 Ed. 2.0: Low-voltage switchgear and controlgear - Part 6-1: Multiple function equipment -
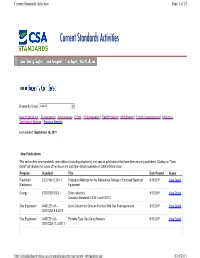
Page 1 of 25 Current Standards Activities 9/16/2011 Http
Current Standards Activities Page 1 of 25 Browse By Group: View All New Publications | Supplements | Amendments | Errata | Endorsements | Reaffirmations | Withdrawals | Formal Interpretations | Informs & Certification Notices | Previous Reports Last updated: September 16, 2011 New Publications This section lists new standards, new editions (including adoptions), and special publications that have been recently published. Clicking on "View Detail" will display the scope of the document and other details available on CSA’s Online store. Program Standard Title Date Posted Scope Electrical / C22.2 No. 0.22-11 Evaluation Methods for Arc Resistance Ratings of Enclosed Electrical 9/15/2011 View Detail Electronics Equipment Energy F378 SERIES-11 Solar collectors 9/12/2011 View Detail Contains Standards F378.1 and F378.2 Gas Equipment ANSI Z21.41- Quick Disconnect Devices For Use With Gas Fuel Appliances 9/12/2011 View Detail 2011/CSA 6.9-2011 Gas Equipment ANSI Z21.63- Portable Type Gas Camp Heaters 9/12/2011 View Detail 2011/CSA 11.3-2011 http://standardsactivities.csa.ca/standardsactivities/recent_infoupdate.asp 9/16/2011 Current Standards Activities Page 2 of 25 Gas Equipment ANSI Z21.72- Portable type gas camp stoves 9/12/2011 View Detail 2011/CSA 11.2-2011 Gas Equipment ANSI Z21.73- Portable type gas camp lights 9/12/2011 View Detail 2011/CSA 11.1-2011 Life Sciences Z94.4-11 Selection, use, and care of respirators 9/12/2011 View Detail Construction CAN/CSA-ISO Welding consumables - Wire electrodes and weld deposits for gas 9/12/2011 View Detail Products and 14341:11 shielded metal arc welding of non alloy and fine grain steels - Materials Classification Adopted ISO 14341:2010, second edition, 2010-02-15 Construction CAN/CSA-ISO Produits consommables pour le soudage - Fils-électrodes et métaux 9/12/2011 View Detail Products and 14341:11 d'apport déposés en soudage à l'arc sous protection gazeuse des aciers Materials non alliés et à grains fins - Classification norme ISO 14341:2010 adoptée, deuxième édition, 2010-02-15 Electrical / CAN/CSA-C22.2 NO. -

Standards for Enabling Trade— Mapping and Gap Analysis Study
Standards for Enabling Trade— Mapping and Gap Analysis Study An IA-CEPA Early Outcomes Initiative November 2017 Standards For Enabling Trade—Mapping and Gap Analysis Study 2 An IA-CEPA Early Outcomes Initiative – November 2017 Contents ListofFigures..............................................................................................................3 Abbreviations...............................................................................................................4 Terms..........................................................................................................................6 Acknowledgements......................................................................................................8 ExplanatoryNotes........................................................................................................8 Foreword.....................................................................................................................9 Recommendations.....................................................................................................10 ExecutiveSummary....................................................................................................11 Introduction................................................................................................................13 ProjectPurpose.........................................................................................................13 Objectives..................................................................................................................13 -
Mitigating Risk with IEC 62304 and Proven Medical Device Software
System Design Considerations for Medical Device Manufacturers Jim McElroy Mark Prowten Director, Medical Director, OEM Embedded Industry Business Wireless, Silex Development, Green Hills Technology America Software © 2011 Green Hills Software, Inc. and Silex Technology , Inc. Slide 1 The Medical Device Manufacturer’s Goal Deliver innovative yet safe, effective and secure medical devices to market cost effectively © 2011 Green Hills Software, Inc. and Silex Technology , Inc. Slide 2 Device Development Challenges Regulatory - Increased oversight, regulation, and most important – Accountability . U.S. Medical Device Accountability Act most definitely will affect how software will be developed in the future . Submissions: FDA 510(k), PMA . Standards: IEC 62304, IEC 60601, ISO 14971, IEC 61508, IEC 62366 Technical - Medical devices are becoming increasingly complex . More capable, more connectivity, requiring less power, smaller footprint, lower cost to produce . More security, reliability, and safety . Many of these forces work against each other ( ex. security and safety vs. innovation and interoperability) © 2011 Green Hills Software, Inc. and Silex Technology , Inc. Slide 3 Client Applications – Connectivity on the Rise Anesthesia Hospital beds Automated External Defibrillator Medical dispensing (AED) Radiography Blood Analyzers Computed Tomography Imaging Ventilator (CT) Dialysis Positron Emission Tomography Infusion pumps Imaging (PET) Glucose monitoring Ultrasound Imaging Patient monitoring Respirator Surgical Navigation Sleep apnea Imaging Magnetic Resonance Imaging (MRI) © 2011 Green Hills Software, Inc. and Silex Technology , Inc. Slide 4 Technical Considerations Device Safety Classification – Class II, III Software Safety Classification under IEC 62304 – Class A, B, or C Security concerns Architecture of the device Leveraging SOUP in medical devices Power and mobility considerations Device Connectivity and Interoperability – Wired and/or Wireless . -

Developing Open-Source Software RTOS with Functional Safety in Mind ? Anas Nashif, Intel Open-Source Technology Center Disclaimer
Developing Open-Source Software RTOS with Functional Safety in Mind ? Anas Nashif, Intel Open-Source Technology Center Disclaimer: I am not a safety expert. Credit: https://unsplash.com/@rawpixel Can open-source software be used for Functional Safety? • Short answer is yes, using open-source software is very common, in all areas • But… • Open-source software usually require major transformation before it can be used • Mostly such transformation happens behind closed doors (if license allows that) • Complete disconnect between original source and “certified” code • Transformation of open-source code to be functionally safe is “expensive” • Following standards very early in a project life-cycle is key • There are many standards… Safety Standards “The nice thing about standards is that there are so many of them to choose from.” [Tanenbaum] Is this possible? Example: RTOS • Open source implementation • Small trusted code base (in terms of LoC) • Safety oriented architecture • Built in security model • POSIX compliant C library • Supports deterministic thread scheduling • Supports multi-core thread scheduling • Proof that ISO compliant development was done • Accountability for the implementation • Industry Adoption • Certification friendly interfaces Cathedral and the Bazaar • Open-source Software is not a problem in itself • It is difficult to map a stereotypical open-source development to the V- model • Specification of features • Comprehensive documentation • Traceability from requirements to source code • Number of committers and information known about them • Certification authority not familiar with open-source development V-Model: Software Development Quality Matters • Quality is a mandatory expectation for software across the industry. ... • Software Quality is not an additional requirement caused by functional .. -

60601 Checklist 1 Intro Rev33
60601 Clause Checklist, Rev. 33 (05/2005) Medical Evaluation Package (IEC 60601-1 with National Deviations) IEC 60601-1, UL 60601-1, CSA C22.2 No. 601.1, EN 60601-1 (With National Deviations) Company: Contact: Product Type: Phone: Model: Email: Ratings: V, Hz, A, W VA Bat. BATTERY INSULATION DIAGRAM Table: to insulation diagram Area Insulation Reference Required Required Measured Measured Dielectric Remarks BOP, BI, SI, voltage creepage clearance creepage clearance Voltage DI/RI (V) (mm) (mm) (mm) (mm) (kV) A B C D E F G H I J K L M N O P MECA - Medical Equipment Compliance Associates, LLC (Download at http://60601-1.com/documents.htm ) Compiled by Brian R. Biersach 1 60601 Clause Checklist, Rev. 33 (05/2005) Medical Evaluation Package (IEC 60601-1 with National Deviations) EVALUATION PROCEDURE: Overall equipment description, accessories to equipment, area(s) equipment will be used , intended power supply, overall objectives ¡ ¡ MECA Project Proposal (Quote) ¡ PO # from Client to open project ¡ Preliminary i nvestigation ¡ Anticipated date samples will be ready for testing ¡ Anticipated equipment production date ¡ UL L-37 & L-40 forms signed Conduct Preliminary Evaluation to Identify Potential Noncompliances and Determine Critical Components and Test Plan ¡ Examine equipment, discuss its use, accessories, interconnection to other equipment, construction materials ¡ Determine if collateral or particular standards are required (See list of Collateral & Particular Standards) ¡ Document Classification of equipment (Per Clause 5) ¡ Examine