Coding Corner: Pacing at the Bundle Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4B. the Heart (Cor) 1
Henry Gray (1821–1865). Anatomy of the Human Body. 1918. 4b. The Heart (Cor) 1 The heart is a hollow muscular organ of a somewhat conical form; it lies between the lungs in the middle mediastinum and is enclosed in the pericardium (Fig. 490). It is placed obliquely in the chest behind the body of the sternum and adjoining parts of the rib cartilages, and projects farther into the left than into the right half of the thoracic cavity, so that about one-third of it is situated on the right and two-thirds on the left of the median plane. Size.—The heart, in the adult, measures about 12 cm. in length, 8 to 9 cm. in breadth at the 2 broadest part, and 6 cm. in thickness. Its weight, in the male, varies from 280 to 340 grams; in the female, from 230 to 280 grams. The heart continues to increase in weight and size up to an advanced period of life; this increase is more marked in men than in women. Component Parts.—As has already been stated (page 497), the heart is subdivided by 3 septa into right and left halves, and a constriction subdivides each half of the organ into two cavities, the upper cavity being called the atrium, the lower the ventricle. The heart therefore consists of four chambers, viz., right and left atria, and right and left ventricles. The division of the heart into four cavities is indicated on its surface by grooves. The atria 4 are separated from the ventricles by the coronary sulcus (auriculoventricular groove); this contains the trunks of the nutrient vessels of the heart, and is deficient in front, where it is crossed by the root of the pulmonary artery. -

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -
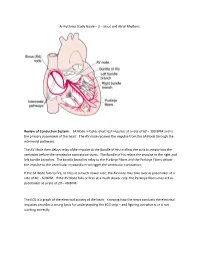
Arrhythmia Study Guide – 2 – Sinus and Atrial Rhythms
Arrhythmia Study Guide – 2 – Sinus and Atrial Rhythms Review of Conduction System: SA Node initiates electrical impulses at a rate of 60 – 100 BPM and is the primary pacemaker of the heart. The AV node recieves the impulse from the SA Node through the internodal pathways. The AV Node then delays relay ofthe impulse to the Bundle of His to allow the atria to empty into the ventricles before the ventricular contraction starts. The Bundle of His relays the impulse to the right and left bundle branches. The bundle branches relay to the Purkinje Fibers and the Purkinje Fibers deliver the impulse to the ventricular myocardium to trigger the ventricular contraction. If the SA Node fails to fire, or fires at a much slower rate, the AV node may take over as pacemaker at a rate of 40 - 60 BPM. If the AV Node fails or fires at a much slower rate, the Perkinjie fibers may act as pacemaker at a rate of 20 – 40 BPM. The ECG is a graph of the electrical activity of the heart. Knowing how the heart conducts the electrical impulses provides a strong basis for understanding the ECG strip – and figuring out what is or is not working correctly. SINUS RHYTHMS Rhythms that originate from the SA Node are characterized by upright uniform P-waves followed by a QRS complex. The P-R interval is constant, and the atrial (P-waves) and ventricular (QRS Complex) rhythms are regular. A rate less than 60 is bradycardic, 60 to 100 is normal, over 100 is tachycardic. -

Ventricular Anatomy for the Electrophysiologist (Part
Ventricular Anatomy for the REVIEW Electrophysiologist (Part II) SPECIAL 서울대학교 의과대학 병리학교실 서정욱 이화여자대학교 의학전문대학원 김문영 ABSTRACT The conduction fibers and Purkinje network of the ventricular myocardium have their peculiar location and immuno-histochemical characteristics. The bundle of His is located at the inferior border of the membranous septum, where the single trunk ramifies into the left and right bundle branches. The left bundle branches are clearly visible at the surface. The right bundles are hidden in the septal myocardium and it is not easy to recognize them. The cellular characters of the conduction bundles are modified myocardial cells with less cytoplasmic filaments. Myoglobin is expressed at the contractile part, whereas CD56 is expressed at the intercalated disc. A fine meshwork of synaptophysin positive processes is noted particularly at the nodal tissue. C-kit positive cells are scattered, but their role is not well understood. Purkinje cells are a peripheral continuation of bundles seen at the immediate subendocardium of the left ventricle. Key words: ■ conduction system ■ Purkinje network ■ pathology ■ arrhythmia ■ electrophysiology Introduction human heart. In this brief review, the histological characteristics of conduction cells, stained by The functional assessment of abnormal cardiac conventional and immuno-histochemical staining, are 3 rhythm and a targeted treatment based on demonstrated in the second part of the review. electrophysiologic studies are successful advances in cardiology.1 Morphological assessment or confirmation The characteristic location of the ventricular of hearts with such abnormalities is rare, not only due conduction system to the limited availability of human hearts but also inherent technological limitations of existing The atrioventricular node is situated in its technology.2 Classical morphological approaches and subendocardial location at the triangle of Koch. -

Cardiology Self Learning Package
Cardiology Self Learning Package Module 1: Anatomy and Physiology of the Module 1: Anatomy and Physiology of the Heart Heart. Page 1 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) CONTENT Introduction…………………………………………………………………………………Page 3 How to use the ECG Self Learning package………………………………………….Page 4 Overview of the Heart…………………………………………………...…………..…….Page 5 Location, Size and Shape of the Heart…………………………………………………Page 5 The Chambers of the Heart…………….………………………………………..……….Page 7 The Circulation System……………………………………….………………..…………Page 8 The Heart Valve Anatomy………………………….…………………………..…………Page 9 Coronary Arteries…………………………………………….……………………..……Page 10 Coronary Veins…………………………………………………………………..……….Page 11 Cardiac Muscle Tissue……………………………………………………………..……Page 12 The Conduction System………………………………………………………………...Page 13 Cardiac Cycle……………………………………………………………………………..Page 15 References…………………………………………………………………………………Page 18 Module Questions………………………………………………………………………..Page 19 Module Evaluation Form………………………………………………………………..Page 22 [Module 1: Anatomy and Physiology of the Heart Page 2 Developed by Tony Curran (Clinical Nurse Educator) and Gill Sheppard (Clinical Nurse Specialist) Cardiology (October 2011) INTRODUCTION Welcome to Module 1: Anatomy and Physiology of the Heart. This self leaning package is designed to as tool to assist nurse in understanding the hearts structure and how the heart works. The goal of this module is to review: Location , size and shape of the heart The chambers of the heart The circulation system of the heart The heart’s valve anatomy Coronary arteries and veins Cardiac muscle tissue The conduction system The cardiac cycle This module will form the foundation of your cardiac knowledge and enable you to understand workings of the heart that will assist you in completing other modules. Learning outcomes form this module are: To state the position of the heart, the size and shape. -

Redalyc.Conduction System in the Swine Heart: Essential Landmarks
Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Sistema de Información Científica Mingsakul, Thaworn; Surachon, Preeyaporn; Somana, Reon Conduction System in the Swine Heart: Essential Landmarks for Gross Dissection Acta Scientiae Veterinariae, vol. 42, núm. 1, enero, 2014, pp. 1-8 Universidade Federal do Rio Grande do Sul Porto Alegre, Brasil Available in: http://www.redalyc.org/articulo.oa?id=289029240048 Acta Scientiae Veterinariae, ISSN (Printed Version): 1678-0345 [email protected] Universidade Federal do Rio Grande do Sul Brasil How to cite Complete issue More information about this article Journal's homepage www.redalyc.org Non-Profit Academic Project, developed under the Open Acces Initiative Acta Scientiae Veterinariae, 2014. 42: 1211. RESEARCH ARTICLE ISSN 1679-9216 Pub. 1211 Conduction System in the Swine Heart: Essential Landmarks for Gross Dissection Thaworn Mingsakul1, Preeyaporn Surachon1 & Reon Somana2 ABSTRACT Background: The components of the cardiac conduction system (CCS) were discovered almost two centuries and presented in the diagrammatic forms. This should be due to the diffi culty in distinguishing the CCS from the surrounding cardiac tissues and the lack of information concerning the precise landmarks for gross dissection. Furthermore the CCS in pig, the animal regarded as a suitable model for the assessment of catheter based intervention, has not been reported. The aims of the present study were to demonstrate the gross anatomic architecture of CCS in the swine heart, and to provide the valuable landmarks for the gross anatomic dissection of the CCS. Materials, Methods & Results: Twenty hearts of adult Large White pigs (Sus Scrofa domesticus) were used. -

PHV Short.Indd
Anatomy & physiology: The heart Cardiac electrical conductance A Sinoatrial (SA) node D Right bundle branch B Atrioventricular (AV) node E Left bundle branch A C Atrioventricular bundle of His F Purkinje fibres The sinoatrial (SA) node is the heart's natural pacemaker, B containing cells that generate electrical impulses which spread F through the atria, triggering contraction of the atria, forcing blood into the ventricles, and stimulating the atrioventricular A (AV) node. E The AV node is linked with the atrioventricular bundle of His C which transmits impulses to the left and right bundle branches and then to the Purkinje fibres which surround the ventricles. The electrical impulses travel through the Purkinje fibres D causing the ventricles to contract and blood forced out of the heart. F Electrocardiogram (ECG) Heart construction The heart is a muscular, cone-shaped organ located behind The ECG traces the course of the cardiac impulse by the sternum and is approximately the same size as the recording the change in electrical potential on the surface of patient's closed fist. the body. Various parts of the ECG are associated with the travel of electrical impulses though the heart. The heart walls are made up the three structures: the pericardium, myocardium and endocardium. The P wave represents atrial depolarisation which causes the atria to contract. The pericardium is a thick fibrous membrane which surrounds the heart. Its function is to anchor the heart and Q is when the impulses arrive at the atrioventricular (AV) prevents over distension (expanding too much). node. The myocardium is the central layer of the heart and is The QRS complex represents ventricular depolarisation and formed from cardiac muscle tissue, it is this muscle which atrial repolarisation (ventricles contract and atria relax and provides the force which pumps the blood around the body. -
Cardiovascular Unit PPT
Cardiovascular Unit PPT Careers Bell ringer: Turn to careers section in Unit packet (last page) fill in Career guided notes • Take a minute and go to each of the 4 Career stations around the room. 1. Read through the pamphlet 2. Fill in Career chart (on last page of unit packet) 3. Read about the career litigation suit on the back of the pamphlet. 4. Move on to another station Heart Anatomy: Flashcards: •You will need to: •Cut •Hole punch •Get 1 color Review Heart Anatomy •Quizlet.live Video segment “The Matter of the Heart”: Watch the first time then take notes Video on teacher website Blood flow coloring: • When finished fill out the questions to the right of coloring in packet. • Try without book, then book Path of Blood: blood flow 3. Right atrium 4. Tricuspid valve 5. Right ventricle 2. superior/inferior vena cava 6. Pulmonary arteries 1. All parts of the body 7. lungs 12. aorta 8.Pulmonary veins 11. Left ventricle 10. bicuspid/mitral 9. Left atrium valve Blood flow: a little more realistically Review Heart Anatomy: Using Heart Models •You and a partner will need a wet pen, cloth eraser, heart model, dry erase pen/eraser •PLEASE: •Label the heart model •Write 4 directional term sentences comparing 2 structures of the heart. Quick Quiz: Make a new Quizlet set: Cardiovascular ADL meds medication am Morning MI Myocardial infarction BLS Basic life support NPO bpm OR B/P, BP preop Before surgery CCU postop After surgery CHD RR CHF CXR DOB Dx ECG/ EKG Etiol Keep quizlet open Shiny desk: Medical abbreviations practice 1. -

22. Heart.Pdf
CARDIOVASCULAR SYSTEM OUTLINE 22.1 Overview of the Cardiovascular System 657 22.1a Pulmonary and Systemic Circulations 657 22.1b Position of the Heart 658 22 22.1c Characteristics of the Pericardium 659 22.2 Anatomy of the Heart 660 22.2a Heart Wall Structure 660 22.2b External Heart Anatomy 660 Heart 22.2c Internal Heart Anatomy: Chambers and Valves 660 22.3 Coronary Circulation 666 22.4 How the Heart Beats: Electrical Properties of Cardiac Tissue 668 22.4a Characteristics of Cardiac Muscle Tissue 668 22.4b Contraction of Heart Muscle 669 22.4c The Heart’s Conducting System 670 22.5 Innervation of the Heart 672 22.6 Tying It All Together: The Cardiac Cycle 673 22.6a Steps in the Cardiac Cycle 673 22.6b Summary of Blood Flow During the Cardiac Cycle 673 22.7 Aging and the Heart 677 22.8 Development of the Heart 677 MODULE 9: CARDIOVASCULAR SYSTEM mck78097_ch22_656-682.indd 656 2/14/11 4:29 PM Chapter Twenty-Two Heart 657 n chapter 21, we discovered the importance of blood and the which carry blood back to the heart. The differences between I myriad of substances it carries. To maintain homeostasis, blood these types of vessels are discussed in chapter 23. Most arteries must circulate continuously throughout the body. The continual carry blood high in oxygen (except for the pulmonary arteries, pumping action of the heart is essential for maintaining blood as explained later), while most veins carry blood low in oxygen circulation. If the heart fails to pump adequate volumes of blood, (except for the pulmonary veins). -

Anatomy and Physiology of the Cardiovascular System
Chapter © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 5 NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Anatomy© Jonesand & Physiology Bartlett Learning, LLC of © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION the Cardiovascular System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION OUTLINE Aortic arch: The second section of the aorta; it branches into Introduction the brachiocephalic trunk, left common carotid artery, and The Heart left subclavian artery. Structures of the Heart Aortic valve: Located at the base of the aorta, the aortic Conduction System© Jones & Bartlett Learning, LLCvalve has three cusps and opens© Jonesto allow blood & Bartlett to leave the Learning, LLC Functions of the HeartNOT FOR SALE OR DISTRIBUTIONleft ventricle during contraction.NOT FOR SALE OR DISTRIBUTION The Blood Vessels and Circulation Arteries: Elastic vessels able to carry blood away from the Blood Vessels heart under high pressure. Blood Pressure Arterioles: Subdivisions of arteries; they are thinner and have Blood Circulation muscles that are innervated by the sympathetic nervous Summary© Jones & Bartlett Learning, LLC system. © Jones & Bartlett Learning, LLC Atria: The upper chambers of the heart; they receive blood CriticalNOT Thinking FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Websites returning to the heart. Review Questions Atrioventricular node (AV node): A mass of specialized tissue located in the inferior interatrial septum beneath OBJECTIVES the endocardium; it provides the only normal conduction pathway between the atrial and ventricular syncytia. -

Morphology of Right Atrioventricular Valve Annulus and Leaflets in Autopsy Specimens
Jemds.com Original Research Article Morphology of Right Atrioventricular Valve Annulus and Leaflets in Autopsy Specimens Raniprabha Sukumaran1, Maheswary Thampi Santhakumary2 1Assistant Professor, Department of Anatomy, Government Medical College, Kottayam, Kerala, India. 2Associate Professor, Department of Anatomy, Government Medical College, Kottayam, Kerala, India. ABSTRACT BACKGROUND Most tricuspid valve conditions are mechanical problems that will require surgery Corresponding Author: Dr. Maheswary Thampi Santhakumary, like valve repair and valve replacement. The proper size of the valve prosthesis and Associate Professor, the size of the annuloplasty band are determined by the measurements of the valve Department of Anatomy, annulus and leaflets. The present study is aimed to provide normal measurements of Government Medical College, the tricuspid valve that would help cardiac surgeons in proper planning and Kottayam, Kerala, India. execution of tricuspid valve surgeries. E-mail: [email protected] METHODS DOI: 10.14260/jemds/2019/552 This descriptive study was conducted in 60 adult human hearts of both sexes during Financial or Other Competing Interests: medico legal autopsy in the Department of Forensic Medicine, GMC Kottayam from None. November 2018 to April 2019. The heart specimens, free from any pathology, variation, were dissected. The diameter, circumference of the annulus and the length How to Cite This Article: and height of the leaflets were measured. The range, mean, standard deviation and Sukumaran R, Santhakumary MT. coefficient of variation of each parameter were calculated. Morphology of right atrioventricular valve annulus and leaflets in autopsy specimens. RESULTS J. Evolution Med. Dent. Sci. 2019;8(32): In the present study, the circumference of the tricuspid valve measured 11.65+/- 1.28 2534-2538, DOI: cms. -

Anatomy of the Heart
Anatomy of the Heart Prof.Musaad Alfayez OBJECTIVES • At the end of the lecture, the student should be able to : • Describe the shape of heart regarding : apex, base, sternocostal and diaphragmatic surfaces. • Describe the interior of heart chambers : right atrium, right ventricle, left atrium and left ventricle. • List the orifices of the heart : • Right atrioventricular (Tricuspid) orifice. • Pulmonary orifice. • Left atrioventricular (Mitral) orifice. • Aortic orifice. • Describe the innervation of the heart • Briefly describe the conduction system of the Heart The Heart • It lies in the middle mediastinum. • It is surrounded by a fibroserous sac called pericardium which is differentiated into an outer fibrous layer (Fibrous pericardium) & inner serous sac (Serous pericardium). The Heart • The Heart is somewhat pyramidal in shape, having: • Apex • Sterno-costal (anterior surface) • Base (posterior surface). • Diaphragmatic (inferior surface) • It consists of 4 chambers, 2 atria (right& left) & 2 ventricles (right& left) Apex of the heart • Directed downwards, forwards and to the left. • It is formed by the left ventricle. • Lies at the level of left 5th intercostal space 3.5 inch from midline. Note that the base of the heart is called the base because the heart is pyramid shaped; the base lies opposite the apex. The heart does not rest on its base; it rests on its diaphragmatic (inferior) surface Sterno-costal (anterior)surface • Divided by coronary (atrio- This surface is formed mainly ventricular) groove into : by the right atrium and the right . Atrial part, formed mainly by ventricle right atrium. Ventricular part , the right 2/3 is formed by right ventricle, while the left l1/3 is formed by left ventricle.