Association Between Coagulation Function and Patients with Primary Angle Closure Glaucoma: a 5-Year Retrospective Case–Control Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Markers of Carotid Atherosclerosis in Type 2 Diabetes Mellitus? Riya M Waghale, Rajashree Sanjay Khot, Prashant P Joshi Impact of Smoking and Nicotine Addiction
2020, Vol. 9, No. 2 ISSN 2450–7458 Platelet volume indices: markers of carotid atherosclerosis in type 2 diabetes mellitus? Riya M Waghale, Rajashree Sanjay Khot, Prashant P Joshi Impact of smoking and nicotine addiction on HbA1c levels and diabetic microvascular complication Hüseyin Akkuzulu, Cenk Aypak, Ayşe Özdemir, Süleyman Görpelioğlu Sugary beverages consumption and latent autoimmune diabetes in adults: systematic review and meta-analysis Ahmed Mahmoud El-Malky, Ramachandra G Naik, Azza A Elnouman The effect of low dose glargine U 300 on uncontrolled type 2 diabetes mellitus. An observational study in Indian patients Asis Mitra, Saswati Ray, Sushma Jayan Coffee in the diet and prevention of diabetes Regina Wierzejska Editor-in-Chief Scientific Board dr hab. n. med. Leszek Czupryniak, prof. nadzw. (Poland) prof. Antionio Ceriello (Spain) prof. dr hab. n. med. Edward Franek (Poland) Deputy Editor-in-Chief prof. dr hab. n. med. Władysław Grzeszczak (Poland) prof. dr hab. n. med. Wojciech Młynarski (Poland) prof. Martin Haluzík (Czech Republic) prof. dr hab. n. med. Krzysztof Strojek (Poland) prof. dr hab. n. med. Przemysława Jarosz-Chobot (Poland) prof. Nebojsa Lalic (Serbia and Montenegro) Editorial Board prof. Pierre Lefebvre (Belgium) prof. dr hab. n. med. Katarzyna Cypryk (Poland) prof. dr hab. n. med. Maciej Małecki (Poland) prof. Larisa Danilova (Belarus) prof. dr hab. n. med. Andrzej Milewicz (Poland) prof. dr hab. n. med. Janusz Gumprecht (Poland) prof. dr hab. n. med. Dariusz Moczulski (Poland) prof. dr hab. n. med. Krzysztof Narkiewicz (Poland) prof. dr hab. n. med. Irina Kowalska (Poland) dr Katherine Owen (United Kingdom) prof. dr hab. n. med. -

Interpreting Your Child's Lab Results
www.ComplexChild.com Interpreting Your Child’s Lab Results When you get a list of labs back from your doctor or hospital, your eye is drawn immediately to the starred or highlighted results that came back abnormal. Knowing that any of your child’s results have come back abnormal can be disconcerting. Much of the time, however, there is no need to worry. But how do you know when abnormal results indicate a little problem, a big problem, or not a problem at all? Reference Ranges The first thing you need to do is look at the reference ranges that are given along with the results. Each lab has its own equipment that is calibrated uniquely, and results vary depending on the lab. While most tests have similar reference ranges from lab to lab, there are some, such as the test for Lipase, that use different testing systems with very different reference ranges. Results from one lab may not be equivalent to results at another lab. The Slightly High or Low Result In many cases, the abnormal result is ever so slightly high or low. Since reference ranges usually represent two standard deviations above or below the average mean value in healthy people, it is still normal for about five percent of the population to be ever so slightly higher or lower than the reference range. Keeping this in mind, a slightly low or high result is rarely concerning. Tests may also be slightly off due to illness, food or drink consumed, or many other factors. Many doctors will advise repeating the tests at a later time to see if the value has normalized, remains the same, or is trending upward or downward. -
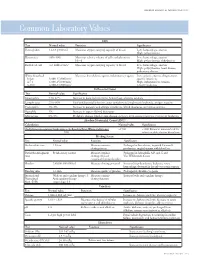
Common Laboratory Values
AmericAn AcAdemy of PediAtric dentistry Reference Manual 2006-2007 Resource Section 251 Common LaboratoryCommon Values Laboratory Values CBC Test Normal value Function Significance Hemoglobin 12-18 g/100 mL Measures oxygen carrying capacity of blood Low: hemorrhage, anemia High: polycythemia Hematocrit 35%-50% Measures relative volume of cells and plasma in Low: hemorrhage, anemia blood High: polycythemia, dehydration Red blood cell 4-6 million/mm3 Measures oxygen-carrying capacity of blood Low: hemorrhage, anemia High: polycythemia, heart disease, pulmonary disease White blood cell Measures host defense against inflammatory agents Low: aplastic anemia, drug toxicity, Infant 8,000-15,000/mm3 specific infections 4-7 y 6,000-15,000/mm3 High: inflammation, trauma, 8-18 y 4,500-13,500/mm3 toxicity, leukemia Differential Count Test Normal value Significance Neutrophils 54%-62% Increase in bacterial infections, hemorrhage, diabetic acidosis Lymphocytes 25%-30% Viral and bacterial infection, acute and chronic lymphocytic leukemia, antigen reaction Eosinophils 1%-3% Increase in parasitic and allergic conditions, blood dyscrasias, pernicious anemia Basophils 1% Increase in types of blood dyscrasias Monocytes 0%-9% Hodgkin’s disease, lipid storage disease, recovery from severe infections, monocytic leukemia Absolute Neutrophil Count (ANC) Calculation Normal value Significance (% Polymorphonuclear Leukocytes + % Bands)×Total White Cell Count >1500 <1000 Patient at increased risk for 100 infection; defer elective dental care Bleeding Screen Test -

Understanding Your Blood Test Lab Results
Understanding Your Blood Test Lab Results A comprehensive "Health Panel" has been designed specifically to screen for general abnormalities in the blood. This panel includes: General Chemistry Screen or (SMAC), Complete Blood Count or (CBC), and Lipid examination. A 12 hour fast from all food and drink (water is allowed) is required to facilitate accurate results for some of the tests in this panel. Below, is a breakdown of all the components and a brief explanation of each test. Abnormal results do not necessarily indicate the presence of disease. However, it is very important that these results are interpreted by your doctor so that he/she can accurately interpret the findings in conjunction with your medical history and order any follow-up testing if needed. The Bernards Township Health Department and the testing laboratory cannot interpret these results for you. You must speak to your doctor! 262 South Finley Avenue Basking Ridge, NJ 07920 www.bernardshealth.org Phone: 908-204-2520 Fax: 908-204-3075 1 Chemistry Screen Components Albumin: A major protein of the blood, albumin plays an important role in maintaining the osmotic pressure spleen or water in the blood vessels. It is made in the liver and is an indicator of liver disease and nutritional status. A/G Ratio: A calculated ratio of the levels of Albumin and Globulin, 2 serum proteins. Low A/G ratios can be associated with certain liver diseases, kidney disease, myeloma and other disorders. ALT: Also know as SGPT, ALT is an enzyme produced by the liver and is useful in detecting liver disorders. -

Prothrombin Time (PT) Be Kept in Constant and Incubation Temperature at Step 3, Start Stop Watch , Mix in a Water Bath ( 37°C ) (LIQUID REAGENT) 36.5-37.5°C
components of any other kit with different lot numbers. temperature 15 minutes prior to testing. Throughout testing all test tubes, syringes and pipettes 2. Pre-warm PT reagent at 37°C for 5 minutes. should be plastic. 3. Pipette 100µl of PT reagent to each tube. Throughout testing all test tubes, incubation time should 4. Add 50 µl of sample, controls to the tubes prepared in Prothrombin Time (PT) be kept in constant and incubation temperature at step 3, start stop watch , mix in a water bath ( 37°C ) (LIQUID REAGENT) 36.5-37.5°C. for 8 seconds , then record the time required for clot For In-Vitro diagnostic use only If testing is delayed for more than 4 hours, plasma may formation . be stored at 2-8°C. B.Automated Method Store at: 2-8°C Each laboratory should establish a Quality Control To perform this test, refer to the appropriate Instrument program that includes both normal and abnormal control Operator’s Manual for detailed instructions. INTENDED USE plasmas to evaluate instrument, reagent tested daily Prothrombin Time (PT) is commonly used for screening for prior to performing tests on patient plasmas. Monthly RESULTS extrinsic factor deficiency, monitoring oral anticoagulant quality control charts are recommended to determine Clot time of the test Prothrombin Time plasma therapy and quantitative determination of the extrinsic the mean and standard deviation of each of the daily = Ratio (PTR) Clot time of the control coagulation factors. control plasma. All assays should include controls, and if plasma any of the controls are outside the established reference INR = PTRISI PRINCIPLE ranges, then the assay should be considered invalid and Example: for a PTR of 2.0 and an ISI of 1.0 Tissue thromboplastin, in the presence of calcium ions and no patient results should be reported. -

Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients
diseases Article Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients Samuel Antwi-Baffour 1,* , Ransford Kyeremeh 1 and Lawrence Annison 2 1 Department of Medical Laboratory Sciences, School of Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB 143 Accra, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Allied Health Sciences, Narh-Bita College, P.O. Box Co1061 Tema, Ghana; [email protected] * Correspondence: s.antwi-baff[email protected] Received: 31 July 2019; Accepted: 18 October 2019; Published: 17 December 2019 Abstract: Sickle cell disease (SCD) is an inherited condition characterized by chronic haemolytic anaemia. SCD is associated with moderate to severe anaemia, hypercoagulable state and inconsistent platelet count and function. However, studies have yielded conflicting results with regards to the effect of anaemia on coagulation in SCD. The purpose of this study was to determine the effect of anaemia severity on selected coagulation parameters of SCD patients. Four millilitres of venous blood samples were taken from the participants (SCD and non-SCD patients) and used for analysis of full blood count and coagulation parameters. Data was analysed using SPSS version-16. From the results, it was seen that individuals with SCD had a prolonged mean PT, APTT and high platelet count compared to the controls. There was also significant difference in the mean PT (p = 0.039), APTT (p = 0.041) and platelet count (p = 0.010) in HbSS participants with severe anaemia. Mean APTT also showed significant difference (p = 0.044) with severe anaemia in HbSC participants. -

Mean Platelet Volume Is Elevated in Patients with Psoriasis Vulgaris
http://dx.doi.org/10.3349/ymj.2015.56.3.712 Original Article pISSN: 0513-5796, eISSN: 1976-2437 Yonsei Med J 56(3):712-718, 2015 Mean Platelet Volume Is Elevated in Patients with Psoriasis Vulgaris Dae Suk Kim,1 Jungsoo Lee,1 Sung Hee Kim,1,2 Soo Min Kim,3 and Min-Geol Lee1,2 1Department of Dermatology, Severance Hospital, Cutaneous Biology Research Institute and 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul; 3Department of Dermatology, National Health Insurance Service Ilsan Hospital, Goyang, Korea. Received: April 22, 2014 Purpose: This retrospective study was done to investigate the mean platelet vol- Revised: June 18, 2014 ume (MPV) level in patients with psoriasis vulgaris and its relationship with dis- Accepted: July 23, 2014 ease severity. Materials and Methods: We undertook a cross-sectional study on Corresponding author: Dr. Min-Geol Lee, 176 patients and 101 healthy controls to examine the association between MPV Department of Dermatology, Severance Hospital, and psoriasis. Various clinical and laboratory parameters were analyzed and com- Cutaneous Biology Research Institute, pared. Results: Platelet distribution width and MPV were significantly higher in Yonsei University College of Medicine, patients with psoriasis than controls. In addition, there was positive correlation be- 50-1 Yonsei-ro, Seodaemun-gu, tween Psoriasis Area Severity Index (PASI) and MPV. When psoriasis patients Seoul 120-752, Korea. were grouped into mild psoriasis (PASI<10) and moderate to severe psoriasis Tel: 82-2-2228-2080, Fax: 82-2-393-9157 E-mail: [email protected] (PASI≥10), the MPV of the latter group was significantly elevated. -

Variations in Platelet Indices Among Healthy Nigerian Population
Asian Hematology Research Journal 1(2): 1-7, 2018; Article no.AHRJ.41255 Variations in Platelet Indices among Healthy Nigerian Population 1* 1 D. Ezigbo, Eyiuche and Omeje, Kosiso 1Department of Medical Laboratory Science, University of Nigeria, Enugu Campus, Enugu State, Nigeria. Authors’ contributions This work was carried out in collaboration between both authors. Author DEE designed the study, performed the statistical analysis, wrote the protocol and wrote the first draft of the manuscript. Author OK managed the analyses of the study. Authors DEE and OK managed the literature searches. Both authors read and approved the final manuscript. Article Information DOI: 10.9734/AHRJ/2018/41255 Editor(s): (1) Massimo Berger, Department of “Cellular Therapy”, Division in Pediatric Onco-Hematology, City of health and Science, Regina Margherita Children Hospital, Turin, Italy. Reviewers: (1) Nagahito Saito, Takagi Hospital, Japan. (2) Sumit Dahal, USA. (3) Aigbogun (Jr) Eric, University of Port Harcourt, Nigeria. Complete Peer review History: http://www.sciencedomain.org/review-history/24684 Received 9th March 2018 Accepted 14th May 2018 Original Research Article th Published 18 May 2018 ABSTRACT Background: The degree of platelet activation may be assessed by platelet indices such as platelet count (PC), mean platelet volume (MPV) and platelet distribution width (PDW). Platelet indices are potentially predictive, diagnostic and prognostic useful markers for platelet-related disorders. Objective: The aim of this study was to evaluate platelet indices in a Nigerian population. Methods: One hundred and eighty-six (186) subjects were enrolled for this study (102 females and 84 males). Thirty (30) of the subjects were ≤30 years, 108 were aged between 30 years-60years while 48 of the subjects were above 60 years. -

Complete Blood Count
Print Entire Test Page 1 of 6 Close Window email this page print this page Complete Blood Count Also known as: CBC, Hemogram, CBC with differential Related tests: Blood smear, Hemoglobin, Hematocrit, Red blood cell (RBC) count, White blood cell (WBC) count, White blood cell differential count, Platelet count At A Glance Why get tested? To determine general health status and to screen for a variety of disorders, such as anemia and infection When to get tested? As part of a routine medical exam or as determined by your doctor Sample required? A blood sample drawn from a vein in the arm or a fingerstick or heelstick (newborns) The Test Sample What is being tested? The Complete Blood Count (CBC) test is an automated count of the cells in the blood. It provides information about the white blood cell (WBC), red blood cell (RBC), and platelet populations present. This information includes the number, type, size, shape, and some of the physical characteristics of the cells. In only a minute or two, the hematology instrument (the machine that is used to run the test) can measure thousands of RBCs, WBCs, and platelets and compare them against established normal ranges. Any abnormalities found are noted, and the clinical laboratory scientist (CLS) running the instrument then uses his or her expertise and experience to accept the automated findings and/or to target the sample for further analysis. In most cases, the automated CBC is very accurate and the test is complete at this point. If, however, there are significant abnormalities in one or more of the cell populations, a blood smear test may be performed. -

Research Article D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis
Hindawi BioMed Research International Volume 2020, Article ID 6159720, 10 pages https://doi.org/10.1155/2020/6159720 Research Article D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis Hui Long,1 Lan Nie,2 Xiaochen Xiang,2 Huan Li,1 Xiaoli Zhang,1 Xiaozhi Fu,1 Hongwei Ren,1 Wanxin Liu,2 Qiang Wang ,2 and Qingming Wu 1,2 1Internal Medicine of Tianyou Hospital, Wuhan University of Science and Technology, Wuhan 430064, China 2Institute of Infection, Immunology and Tumor Microenvironment, Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Medical College, Wuhan University of Science and Technology, Wuhan 430065, China Correspondence should be addressed to Qiang Wang; [email protected] and Qingming Wu; [email protected] Received 22 April 2020; Accepted 19 May 2020; Published 17 June 2020 Academic Editor: Frederick D. Quinn Copyright © 2020 Hui Long et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objective. To investigate the value of coagulation indicators D-dimer (DD), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (Fg) in predicting the severity and prognosis of COVID-19. Methods. A total of 115 patients with confirmed COVID-19, who were admitted to Tianyou Hospital of Wuhan University of Science and Technology between January 18, 2020, and March 5, 2020, were included. The dynamic changes of DD, PT, APTT, and Fg were tested, and the correlation with CT imaging, clinical classifications, and prognosis was studied. -

Thrombophilia Testing Panel Now Available
Thrombophilia Testing Panel Now Available Bronson Laboratory offers a any impact on the patient’s therapy. Importantly, most people with an thrombophilia testing panel consisting For instance, the vast majority of older inheritable thrombophilic mutation will of the following: factor VIII activity, anti- patients with venous thromboses in the never experience a thrombotic event, thrombin III activity, protein setting of a recognized inciting event and most of those who do will never C activity, protein S antigen, factor V Leiden will not have a preexisting thrombophilic do so again. Because of this, screening mutation assay, prothrombin 90210A condition, and accordingly testing is the general population or even asympto- mutation assay, and a lupus anticoagulant generally not recommended in this pop- matic relatives of individuals with thrombo- assay. Abnormal values for protein C, ulation. In regards to arterial thromboses, philic mutations is discouraged because protein S, and antithrombin III will be the overwhelming majority are a direct of the potential for harmful overtreatment. automatically reflexed to confirmatory consequence of atherosclerosis, and the By similar reasoning, some hematologists testing at the Mayo Clinic laboratories. added presence of a thrombophilia in would recommend the same period of Although these tests are orderable indi- this setting will not affect patient anticoagulation, often 3-6 months after vidually, there is little rationale to do so management. (continued on page 4) except as noted below. Clinical circumstances that suggest The activity assays are clotting tests a laboratory evaluation for thrombophilia that require adequate amounts of func- tional protein to achieve a normal result. For reasons having to do with greater achievable precision, the free protein S Patient with a Asymptomatic patients Other clinical situations with a family history of VTE antigen test is used in lieu of an activity confirmed acute VTE assay, but it too depends on adequate amounts of circulating protein for a normal finding. -

Three Neglected Numbers in the CBC: the RDW, MPV, and NRBC Count
REVIEW JORI E. MAY, MD MARISA B. MARQUES, MD VISHNU V.B. REDDY, MD RADHIKA GANGARAJU, MD Department of Medicine, Department of Pathology, Department of Pathology, Department of Medicine, University of University of Alabama, University of Alabama, Birmingham University of Alabama, Birmingham Alabama, Birmingham Birmingham Three neglected numbers in the CBC: The RDW, MPV, and NRBC count ABSTRACT he complete blood cell count (CBC) T is one of the most frequently ordered lab- The complete blood cell count (CBC) is one of the most oratory tests in both the inpatient and outpa- frequently ordered laboratory tests, but some values tient settings. Not long ago, the CBC required included in the test may be overlooked. This brief re- peering through a microscope and counting view discusses 3 potentially underutilized components the red blood cells, white blood cells, and of the CBC: the red blood cell distribution width (RDW), platelets. These 3 numbers are still the primary the mean platelet volume (MPV), and the nucleated purpose of the test. red blood cell (NRBC) count. These results have unique Now, with automated counters, the CBC diagnostic applications and prognostic implications that report also contains other numbers that delin- can be incorporated into clinical practice. By understand- eate characteristics of each cell type. For ex- ing all components of the CBC, providers can learn more ample: about the patient’s condition. The mean corpuscular volume is the aver- age volume of red blood cells. Providers use it KEY POINTS to classify anemia as either microcytic, normo- The RDW can help differentiate the cause of anemia: eg, cytic, or macrocytic, each with its own differ- a high RDW suggests iron-defi ciency anemia, while a nor- ential diagnosis.