Brissopsis Ottnangensis HOERNES, 1875
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zu Den Hydrogeologischen Verhältnissen Im Obermarkersdorfer Becken
©Geol. Bundesanstalt, Wien; download unter www.geologie.ac.at Arbeitstagung Geologische Bundesanstalt 1999 • Retz - Hollabrunn Beiträge ZU DEN HYDROGEOLOGISCHEN VERHÄLTNISSEN IM OBERMARKERSDORFER BECKEN Gerhard SCHUBERT Einleitung Das Obermarkersdorfer Becken ist eine kristallinnahe Randbucht der Molassezone. Der na mensgebende Ort befindet sich 4 km südwestlich des Tagungsortes Retz (Abb. 1). Das sich SW-NE-erstreckende Becken besitzt eine Länge von 7 km, die Breite beträgt 3,5 km. Nach Westen, Norden und Osten wird es von kristallinen Aufragungen begrenzt: Im Westen verläuft die parallel zur Diendorfer Störung SW-NE-streichende Waitzendorfer Störung, an die der bis etwa 500 m ü. A. hohe Kristallinrücken des Kohlbergs anschließt. Dieser steht im Nor den mit der N-S-verlaufenden kristallinen Hochzone Retz - Zellerndorf in Verbindung, die mit ihren bis etwa 300 m ü. A. hohen Kristallinkuppen den östlichen Rahmen des Obermarkers dorfer Beckens bildet. Im Süden wird das Verflachen des Beckens durch die westlich Rohren dorf zutage tretenden basalen Sedimente angedeutet. Im Rahmen des seit 1994 laufenden Bund-Bundesländer-Kooperationsprojekts N-C-036 („Na turraumpotential der Bezirke Hörn und Hollabrunn") kristallisierte sich im Themenkreis Hydro geologie (SCHUBERT, 1998) das Obermarkersdorfer Becken als Schwerpunkt heraus. Eine detaillierte Bearbeitung dieses Raumes erschien vor allem deshalb vielversprechend, da sei tens der NÖSIWAG zu ihren im Süden des Beckens befindlichen Brunnenfeldern Pulkau I und II bereits zahlreiche Untersuchungsergebnisse vorlagen. Da zur Zeit das Erkundungsprogramm der NÖSIWAG (Bohrungen, Pumpversuche etc.) und die Quellbeobachtung des Projekts N-C-036 noch nicht abgeschlossen sind, ist die folgende Darstellung der hydrogeologischen Verhältnisse noch als vorläufig zu betrachten. Klimatische Verhältnisse Die klimatischen Verhältnisse im Projektsgebiet lassen sich am anschaulichsten durch die Meßstellen Retz (Abb. -

Satzung Des Gemeindeverbandes Für Abfallwirtschaft Und Abgabeneinhebung Im Verwaltungsbezirk Hollabrunn; Fassung 1
Satzung des Gemeindeverbandes für Abfallwirtschaft und Abgabeneinhebung im Verwaltungsbezirk Hollabrunn; Fassung 1. Jänner 2017 Genehmigt mit der am 20. Dezember 2016 ausgegebenen Novelle der NÖ Gemeinde- verbändeverordnung, LGBl. Nr. 98/2016 SATZUNG §1 Name und Sitz des Gemeindeverbandes Der Gemeindeverband führt den Namen „Gemeindeverband für Abfallwirtschaft und Abgabeneinhebung im Verwaltungsbezirk Hollabrunn“ und hat seinen Sitz in Hollabrunn. §2 Beteiligte Gemeinden Dem Gemeindeverband gehören folgende Gemeinden an: Alberndorf im Pulkautal, Göllersdorf, Grabern, Guntersdorf, Hadres, Hardegg, Haugsdorf, Heldenberg, Hohenwarth-Mühlbach a. M., Hollabrunn, Mailberg, Maissau, Nappersdorf- Kammersdorf, Pernersdorf, Pulkau, Ravelsbach, Retz, Retzbach, Schrattenthal, Seefeld - Kadolz, Sitzendorf an der Schmida, Wullersdorf, Zellerndorf, Ziersdorf. §3 Aufgaben des Gemeindeverbandes (1) Aus dem eigenen Wirkungsbereich der verbandsangehörigen Gemeinden obliegt dem Gemeindeverband die Vollziehung und die Besorgung der Aufgaben auf dem Gebiet der Abfallwirtschaft sowie die Bemessung, Vorschreibung, Einhebung und zwangsweise Einbringung der diesbezüglichen Abgaben und die Beteiligung an Gesellschaften des Handelsrecht, die die Entsorgung und Verwertung von Abfall zum Gegenstand haben. Dem Gemeindeverband obliegt nicht die Sicherung und die Sanierung von Altlasten im Sinne des Altlastensanierungsgesetzes. (2) Aus dem eigenen Wirkungsbereich der verbandsangehörigen Gemeinden obliegt dem Gemeindeverband die Berechnung, Vorschreibung, Einhebung und -

Industrie + Gewerbe Landwirtschaft Und Tourismus Interkommunale Kooperationen Das Tor Zur Wirtschaft
– Standort Ziersdorf – INDUSTRIE + GEWERBE DAS TOR ZUR WIRTSCHAFT Optimaler Standort für Gewerbe- und Der Wirtschaftspark Schmidatal-Manhartsberg Industriebetriebe. Profitieren Sie von liegt im Herzen des Weinviertels – hier treffen sofort verfügbaren gewidmeten und aufgeschlossene Gewerbegründe mit Breitband- vollaufgeschlossenen Gewerbegründen. anschluss auf Kleinkunst und Kellergassen. Die Anbindung an leistungsfähige Straßen > HOHE LEBENSQUALITÄT und dem Schienenverkehr bietet beste > AUSGEZEICHNETE STANDORTQUALITÄT Infrastruktur und perfekte Logistik. > AUSGEZEICHNETES ARBEITSKRÄFTEPOTENZIAL > INTERESSANTER ABSATZMARKT LANDWIRTSCHAFT IM UMFELD UND TOURISMUS > 50 KM ZUR MILLIONENSTADT WIEN Herausragende Qualität der Region mit Authentizität in der Landwirtschaft, Gesamtangebote in der Erlebnis- Wien – B4-Umfahrung Ziersdorf – Horn gastronomie, von Produktion und Ver- BB ! edelung bis zum Konsum. Erreichen Sie Ra Knoten d l b Ziersdorf r neue Zielgruppen im Bereich Tourismus. u e n Mitt n – K r em s e BB rs t ra BB s s e – Z i e ERWEITERUNG MÖGLICH r sd INTERKOMMUNALE o BB r f BS KOOPERATIONEN BS BB Bauland, Betriebsgebiet VP BS Bauland, Sondergebiet Gastronomie VP Private VF Tankstelle Die Betreibergemeinden des Wirt- Gfrei Grünland-Freihaltefläche frei Gfrei BB verkauft schaftsparks setzen im Interesse der regionalen Lebensqualität Kooperations- > Expansionsfläche: 17-19 Hektar möglich projekte um. Mit dem regionalen Post- > Kaufpreis: € 12,80 bis € 14,80 pro m2 verteilerzentrum und dem Abfallzentrum > Techn. Infrastruktur: Strom, Kanal,Znaim Trinkwasser, Gmünd wurden sichtbare Zeichen gesetzt. Breitbandinternet, Telekommunikation,Retz Kläranlage Pulkau Horn > UnternehmenEggenburg vor Ort: SBI Produktion techn. Laa an der Thaya Anlagen GmbH. & Co KG, Post Zustellbasis, Abfallverband Sitzendorf Zwettl Maissau Limberg Ravelsbach Hollabrunn ZIERSDORF Ernstbrunn Glaubendorf Hohenwarth Heldenberg Goßweikersdorf Langenlois Schmid Krems Kirchberg 4 a Stockerau Donau A22 3 Tulln WIEN anz-Josefs-Bahn St. -

AGREEMENT Between the European Community and the Republic Of
L 28/4EN Official Journal of the European Communities 30.1.2002 AGREEMENT between the European Community and the Republic of South Africa on trade in wine THE EUROPEAN COMMUNITY, hereinafter referred to as the Community, and THE REPUBLIC OF SOUTH AFRICA, hereinafter referred to as South Africa, hereinafter referred to as the Contracting Parties, WHEREAS the Agreement on Trade, Development and Cooperation between the European Community and its Member States, of the one part, and the Republic of South Africa, of the other part, has been signed on 11 October 1999, hereinafter referred to as the TDC Agreement, and entered into force provisionally on 1 January 2000, DESIROUS of creating favourable conditions for the harmonious development of trade and the promotion of commercial cooperation in the wine sector on the basis of equality, mutual benefit and reciprocity, RECOGNISING that the Contracting Parties desire to establish closer links in this sector which will permit further development at a later stage, RECOGNISING that due to the long standing historical ties between South Africa and a number of Member States, South Africa and the Community use certain terms, names, geographical references and trade marks to describe their wines, farms and viticultural practices, many of which are similar, RECALLING their obligations as parties to the Agreement establishing the World Trade Organisation (here- inafter referred to as the WTO Agreement), and in particular the provisions of the Agreement on the Trade Related Aspects of Intellectual Property Rights (hereinafter referred to as the TRIPs Agreement), HAVE AGREED AS FOLLOWS: Article 1 Description and Coding System (Harmonised System), done at Brussels on 14 June 1983, which are produced in such a Objectives manner that they conform to the applicable legislation regu- lating the production of a particular type of wine in the 1. -

Bezirksfeuerwehrkommando Hollabrunn
Bezirksfeuerwehrkommando Hollabrunn Josef Weislein-Straße 19 | 2020 Hollabrunn Telefon: +43 (2952) 2222 - 70 | Fax: +43 (2952) 2222 - 71 | EMail: [email protected] endgültige Ergebnisliste Bezirksfeuerwehrleistungsbewerb 25.06.2011 - 25.06.2011 BFKDO Hollabrunn Rang Gruppenname Instanz AFKDO Nr. Gesamt Bronze ohne Alterspunkte / Eigene 1 Riegersburg 1 Riegersburg Retz 12 405,80 2 Thern 1 Thern Ravelsbach 44 405,10 3 Grübern Grübern Ravelsbach 40 400,10 4 Watzelsdorf 1 Watzelsdorf Retz 2 399,60 5 Alberndorf Alberndorf Haugsdorf 20 398,80 6 Kleinriedenthal Kleinriedenthal Retz 4 398,10 7 Zellerndorf 1 Zellerndorf Retz 1 395,40 8 Retz 1 Retz Retz 8 394,80 9 Watzelsdorf 3 Watzelsdorf Retz 14 393,70 10 Obergrabern Obergrabern Hollabrunn 84 389,50 11 Maissau 2 Maissau Ravelsbach 41 385,90 12 Rafing Rafing Retz 7 385,70 13 Jetzelsdorf 1 Jetzelsdorf Haugsdorf 22 381,50 14 Kammersdorf Kammersdorf Hollabrunn 33 381,20 15 Untermarkersdorf Untermarkersdorf Haugsdorf 29 380,80 16 Zellerndorf 2 Zellerndorf Retz 13 379,60 17 Pfaffendorf-Karlsdorf 1 Pfaffendorf-Karlsdorf Haugsdorf 27 379,40 18 Grund Grund Hollabrunn 83 377,80 19 Obergrub Obergrub Hollabrunn 35 377,00 20 Pernersdorf Pernersdorf Haugsdorf 26 374,90 21 Breitenwaida Breitenwaida Hollabrunn 31 373,60 22 Haugsdorf Haugsdorf Haugsdorf 21 371,00 23 Seefeld-Kadolz Seefeld-Kadolz Haugsdorf 28 366,10 24 Eitzersthal I Eitzersthal Hollabrunn 32 365,60 25 Peigarten Peigarten Haugsdorf 82 365,30 26 Retz 2 Retz Retz 15 362,40 27 Ravelsbach Ravelsbach Ravelsbach 43 362,40 28 Groß Wetzdorf Groß -

Moving Wachau, © Robert Herbst
REFRESHINGLY moving Road map of Lower Austria, with tips for visitors WWW.LOWER-AUSTRIA.INFO Mostviertel, © Robert Herbst Mostviertel, Welcome! “With this map, we want to direct you to the most beautiful corners of Lower Austria. As you will see, Austria‘s largest federal state presents itself as a land of diversity, with a wide variety of landscapes for refreshing outdoor adventures, great cultural heritage, world-class wines and regional specialities. All that’s left to say is: I wish you a lovely stay, and hope that your time in Lower Austria will be unforgettable!” JOHANNA MIKL-LEITNER Lower Austrian Governor © NLK/Filzwieser “Here you will find inspiration for your next visit to, or stay in, Lower Austria. Exciting excursion destinations, varied cycling and mountain biking routes, and countless hiking trails await you. This map also includes lots of tips for that perfect stay in Lower Austria. Have fun exploring!” JOCHEN DANNINGER Lower Austrian Minister of Economics, Tourism and Sports © Philipp Monihart Wachau, © Robert Herbst Wachau, LOWER AUSTRIA 2 national parks in numbers Donau-Auen and Thaya Valley. 1 20 Vienna Woods nature parks years old is the age of the Biosphere Reserve. in all regions. Venus of Willendorf, the 29,500 world’s most famous figurine. fortresses, castles 70 and ruins are open to visitors. 93 centers for alpine abbeys and monasteries have “Natur im Garten” show gardens 9 adventure featuring 15 shaped the province and ranging from castle and monastic summer and winter its culture for centuries, gardens steeped in history sports. Melk Abbey being one to sweeping landscape gardens. -

Abwanderungsgemeinden in NÌ Homepage.Xlsx
Abwanderungsgemeinden in Niederösterreich * Kategorie I = Abwanderung von minus 2,5% bis 4,9% X Kategorie II = Abwanderung minus 5% und mehr Aggsbach * Gnadendorf * Kottes-Purk x Puchenstuben * Sigmundsherberg * Albrechtsberg an der Großen Krems* Göstling an der Ybbs x Laab im Walde * Raabs an der Thaya x Sonntagberg * Allentsteig x Grafenbach-Sankt Valentin * Langau x Raach am Hochgebirge x Sooß x Altmelon * Groß Gerungs * Leitzersdorf * Rabensburg * Spitz * Amaliendorf-Aalfang * Großgöttfritz * Lengenfeld * Randegg * Staatz x Annaberg x Großharras x Lichtenegg * Ravelsbach x Statzendorf * Arbesbach x Großriedenthal x Lichtenwörth x Reichenau an der Rax x Stronsdorf * Aspangberg-Sankt Peter * Groß-Schweinbarth * Lilienfeld x Reingers x Ternitz * Bad Großpertholz x Groß-Siegharts * Litschau * Retzbach x Thaya * Bad Schönau * Grünbach am Schneeberg x Loich * Ringelsdorf-Niederabsdorf x Traisen x Bärnkopf x Günselsdorf * Ludweis-Aigen x Rohr im Gebirge * Trattenbach * Brand-Nagelberg x Gutenbrunn x Lunz am See x Rohrbach an der Gölsen x Türnitz * Breitenstein x Gutenstein x Mailberg x Röhrenbach x Velm-Götzendorf * Buchbach * Hardegg x Mannsdorf an der Donau x Rosenburg-Mold x Waidhofen an der Thaya x Dietmanns x Haugschlag * Maria Laach am Jauerling * Rossatz-Arnsdorf x Waidmannsfeld x Dobersberg x Haugsdorf x Martinsberg x Sallingberg x Waldhausen x Dorfstetten x Heidenreichstein x Meiseldorf x Sankt Aegyd am Neuwalde x Waldkirchen an der Thaya x Drosendorf-Zissersdorf * Hirschbach * Mitterbach am Erlaufsee x Sankt Anton an der Jeßnitz -

Climate Star 2021
Klimabündnis Bezirk Climate Star 2021 European Municipalities Compete for Climate Protection CATEGORY 1 – Energy & climate CATEGORY 4 – Everyday sustainability ASCHA (Germany) ....................…………………………… 5 BADEN (Switzerland) ..............................................10 EPPAN (Italy) ��������������������������������������������������������������5 LUDWIGSBURG (Germany) ....................................11 GRAZ (Austria) �����������������������������������������������������������6 PERCHTOLDSDORF (Austria) .................................11 TULLN AN DER DONAU (Austria) ...........................6 DISTRICT OF STEINFURT (Germany) ....................12 TÂRGU MUREȘ (Romania) .....................................12 CATEGORY 2 – Involving citizens HERZOGENBURG (Austria) ............………………………7 Exhibition STATE OF RHINELAND-PALATINATE (Germany) ���7 “CLIMATE & I” TO GO ON TOUR ..........................13 RETZER LAND (Austria) ............................................8 CATEGORY 3 – Saving resources ESCH-SUR-ALZETTE (Luxembourg) …………………… 8 JUNGLINSTER (Luxembourg) ..................................9 KAJÁRPÉC (Hungary) ................................................9 VILLACH (Austria) ���������������������������������������������������10 UWZ_Vermerk_GmbH_4C_Umweltzeichen_Vermerk.qxd 31.05.13 08:02 Seite 1 gedruckt nach der Richtlinie „Druckerzeugnisse“ des Österreichischen Umweltzeichens Druckerei Janetschek GmbH · UW-Nr. 637 Imprint and Legal Notice: gedruckt nach der Richtlinie „Druckerzeugnisse“ des EDITOR, PUBLISHER & MEDIA HOLDER: -

The Styrian Basin: a Key to the Middle Miocene (Bade- Nian/Langhian) Central Paratethys Transgressions___
Austrian Journal of Earth Sciences Volume 102 Vienna 2009 The Styrian Basin: a key to the Middle Miocene (Bade- nian/Langhian) Central Paratethys transgressions___ Johann HOHENEGGER1)*), Fred RÖGL2), Stjepan ĆORIĆ3), Peter PERVESLER1), Fabrizio LIRER4), Reinhard ROETZEL3), Robert SCHOLGER5) & Karl STINGL5) 1) University of Vienna, Department of Palaeontology, Althanstrasse 14, A 1090 Wien, Austria. KEYWORDS 2) Natural History Museum Vienna, Burgring 7, A 1010 Wien, Austria. Badenian Transgressions Chronostratigraphy 3) Austrian Geological Survey, Neulinggasse 38, A 1030 Wien, Austria. Central Paratethys 4) Istituto Ambiente Marino Costiero – CNR, Calata Porta di Massa, interno Porto di Napoli, I-80133 Napoli, Italia. Middle Miocene Styrian Phase 5) University of Leoben, Department of Geosciences and Geophysics, A 8700 Leoben, Austria. Styrian Basin *) Corresponding author, [email protected] Abstract In the Styrian Basin, early Miocene marine sedimentation of the Karpatian (upper Burdigalian) ended with basin shallowing, mar- ked regression and tectonic movements. The Karpatian sedimentation cycle corresponds to the global 3rd order cycle TB 2.2, follo- wed by the Bur5/Lan1 sea-level fall. This regression was combined with tectonic movements (the Styrian Tectonic Phase), seen in the Styrian Unconformity by an angular discordance at the Wagna and Katzengraben outcrops and also in deep wells. Sediments of the first middle Miocene (Badenian/Langhian) transgression are commonly eroded or reduced in thickness at the basin borders. In the basin center, the bathyal environment continues from the Karpatian to the Badenian. Sediments of the first Badenian transgres- sion have been dated by calcareous nannoplankton as NN4 and correlated by the occurrence of Praeorbulina sicana with the basal Langhian. -
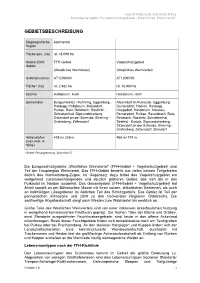
Gebietsbeschreibung
HAUPTREGION WEINVIERTEL Managementplan Europaschutzgebiete „Westliches Weinviertel“ GEBIETSBESCHREIBUNG Biogeografische kontinental Region Fläche ges. (ha) rd. 18.099 ha Natura 2000- FFH-Gebiet Vogelschutzgebiet Gebiet (Westliches Weinviertel) (Westliches Weinviertel) Gebietsnummer AT1209A00 AT1209V00 Fläche* (ha) rd. 2.982 ha rd. 16.904 ha Bezirke Hollabrunn, Horn Hollabrunn, Horn Gemeinden Burgschleinitz - Kühnring, Eggenburg, Alberndorf im Pulkautal, Eggenburg, Hardegg, Hollabrunn, Meiseldorf, Guntersdorf, Hadres, Hardegg, Pulkau, Retz, Retzbach, Röschitz, Haugsdorf, Hollabrunn, Maissau, Schrattenthal, Sigmundsherberg, Pernersdorf, Pulkau, Ravelsbach, Retz, Sitzendorf an der Schmida, Straning - Retzbach, Röschitz, Schrattenthal, Grafenberg, Zellerndorf Seefeld - Kadolz, Sigmundsherberg, Sitzendorf an der Schmida, Straning - Grafenberg, Zellerndorf, Ziersdorf Höhenstufen 438 m/ 226 m 468 m/ 191 m (max./min. m Höhe) * Quelle: Feinabgrenzung, Stand Mai 07 Die Europaschutzgebiete „Westliches Weinviertel” (FFH-Gebiet + Vogelschutzgebiet) sind Teil der Hauptregion Weinviertel. Das FFH-Gebiet besteht aus vielen kleinen Teilgebieten östlich des Manhartsberg-Zuges. Im Gegensatz dazu bildet das Vogelschutzgebiet ein weitgehend zusammenhängendes und deutlich größeres Gebiet, das sich bis in das Pulkautal im Norden ausdehnt. Das Gesamtgebiet (FFH-Gebiet + Vogelschutzgebiet) hat Anteil sowohl an der Böhmischen Masse mit ihren sauren, silikatischen Gesteinen, als auch an kalkhältigen Lössgebieten im östlichen Teil des Schutzgebiets. Das Gebiet ist -
BILDUNGS PROGRAMM Frühjahr/Sommer 2020
- Hühner Buch- haltung Reparatur haltung II Café 2. Weinviertler BILDUNGS PROGRAMM Frühjahr/Sommer 2020 ZUMBA Sirup- herstellung Fitness Töpfern CERN dem Ursprung -des Gärten Universums auf klimafit Smartphone der Spur für Senioren machen Schnaps - Intensivseminar brennen für meinen gesunden Infektionen Rücken bei Kindern 200 Angebote aus 24 Gemeinden im Überblick www.wissbegierig.at Herausgeber und Information: LEADER Region Weinviertler-Manhartsberg Ausstellungsstraße 6, 2020 Hollabrunn 02952 / 305 25, [email protected] www.leader.co.at ÜBER UNS VORWORT Die Entwicklung der Region liegt an uns allen! copyright weinfranz.com Regional gemeinsam fördern Liebe Bildungsinteressierte! LEADER ist das Förderprogramm der Europäischen Bildung macht glücklich. Ganz offensichtlich trifft das Union zur Entwicklung des ländlichen Raumes. Das auf Bildungsangebote wie Töpfern und Theaterspielen, zentrale Ziel ist, dass die Menschen vor Ort ihre Region Lauftreffs und Yoga-Kurse zu. Aber auch der Besuch mitentwickeln. Deswegen wurde in jeder LEADER von Vorträgen und Weiterbildungen macht glücklich: Region eine Zukunftsstrategie erarbeitet, an der Vertre- Mit jedem neuen Thema, dem wir uns stellen, mit jeder terInnen von Gemeinden, Unternehmen, landwirtschaft- Fertigkeit, die wir uns aneignen, erweitern wir unseren lichen Betrieben, uva. mitgewirkt haben. Bewegungsspielraum und wächst unser Potential. Durch das Gelernte und durch das Lernen an sich! Seit 1995 wurden mit diesem Programm in vier mehr- jährigen Förderperioden ca. 530 Projekte umgesetzt. Die Auch wir sind glücklich, weil es uns zum zweiten Mal derzeitige Phase läuft noch bis 2020, im gleichen Jahr gelungen ist, ein Semesterprogramm herauszugeben, wird auch mit der Planung der nächsten Periode gestar- das ebendiese Angebote, Workshops, Vorträge und Kurse tet. bekannter Institutionen und privater AnbieterInnen bündelt – und das für eine ganze Region! Möglich ist das Vielfältige Themen und Projekte umgesetzt durch viel Zeit und Einsatz aller Beteiligten. -

From the Palaeontological Collection of the Provincial Museum Joanneum – the Fossil Crocodylians (Crocodylia) Aus Der Paläont
Joannea Geol. Paläont. 10: 91-125 (2008) From the Palaeontological Collection of the Provincial Museum Joanneum – The Fossil Crocodylians (Crocodylia) Aus der paläontologischen Sammlung des Landesmuseums Joanneum – Die fossilen Krokodile (Crocodylia) Martin GROSS & Jeremy MARTIN 12 Figures Abstract: The crocodylian remains stored in the collection of the Provincial Museum Joanneum (Graz, Austria) are reviewed. Previous descriptions, geographical and strati- graphical provenance and collection history are discussed. The most important area from which these fossils come from – the Wies-Eibiswald coal-mining district – is brief- ly discussed. Preliminary taxonomical considerations concerning the type material of Enneodon ungeri PRANGNER, 1845, Diplocynodon steineri (HOFMANN, 1887a) and Diplo- cynodon styriacus (HOFMANN, 1887a) are provided. Zusammenfassung: Im vorliegenden Katalog werden die am Landesmuseum Joan- neum verwahrten fossilen Krokodilreste hinsichtlich früherer Bearbeitungen, ihrer geo- graphischen und stratigraphischen Position sowie ihrer Sammlungsgeschichte darge- stellt. In einer kurzen Übersicht wird das wichtigste Fundgebiet – das Wies-Eibiswalder Kohlerevier – behandelt sowie der taxonomische Status des Typusmaterials von Enneo- don ungeri PRANGNER, 1845, Diplocynodon steineri (HOFMANN, 1887a) und Diplocyno- don styriacus (HOFMANN, 1887a) evaluiert. Key Words: Diplocynodontidae; Diplocynodon/Enneodon; Styria/Austria; Wies-Eibis- wald; Middle Miocene. Schlüsselworte: Diplocynodontidae; Diplocynodon/Enneodon; Steiermark/Österreich; Wies-Eibiswald; Mittel-Miozän. 91 Contents 1. Introduction . 92 2. The fossil crocodylians at the Joanneum . 94 2.1. Crocodylia – Some background information . 94 2.2. Remarks on the geology and stratigraphy of the Wies-Eibiswald coal-mining district . 96 3. Catalogue of fossil crocodylians at the Joanneum . 99 Acknowledgements . 121 References . 121 1. Introduction The current work aims to bring fossil material stored at the Joanneum to a greater scien- tific audience via printed and online publication (see GROSS 2002).