The Strength of Negative Plant–Soil Feedback Increases from the Intraspecific to the Interspecific and the Functional Group Level
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxa Named in Honor of Ihsan A. Al-Shehbaz
TAXA NAMED IN HONOR OF IHSAN A. AL-SHEHBAZ 1. Tribe Shehbazieae D. A. German, Turczaninowia 17(4): 22. 2014. 2. Shehbazia D. A. German, Turczaninowia 17(4): 20. 2014. 3. Shehbazia tibetica (Maxim.) D. A. German, Turczaninowia 17(4): 20. 2014. 4. Astragalus shehbazii Zarre & Podlech, Feddes Repert. 116: 70. 2005. 5. Bornmuellerantha alshehbaziana Dönmez & Mutlu, Novon 20: 265. 2010. 6. Centaurea shahbazii Ranjbar & Negaresh, Edinb. J. Bot. 71: 1. 2014. 7. Draba alshehbazii Klimeš & D. A. German, Bot. J. Linn. Soc. 158: 750. 2008. 8. Ferula shehbaziana S. A. Ahmad, Harvard Pap. Bot. 18: 99. 2013. 9. Matthiola shehbazii Ranjbar & Karami, Nordic J. Bot. doi: 10.1111/j.1756-1051.2013.00326.x, 10. Plocama alshehbazii F. O. Khass., D. Khamr., U. Khuzh. & Achilova, Stapfia 101: 25. 2014. 11. Alshehbazia Salariato & Zuloaga, Kew Bulletin …….. 2015 12. Alshehbzia hauthalii (Gilg & Muschl.) Salariato & Zuloaga 13. Ihsanalshehbazia Tahir Ali & Thines, Taxon 65: 93. 2016. 14. Ihsanalshehbazia granatensis (Boiss. & Reuter) Tahir Ali & Thines, Taxon 65. 93. 2016. 15. Aubrieta alshehbazii Dönmez, Uǧurlu & M.A.Koch, Phytotaxa 299. 104. 2017. 16. Silene shehbazii S.A.Ahmad, Novon 25: 131. 2017. PUBLICATIONS OF IHSAN A. AL-SHEHBAZ 1973 1. Al-Shehbaz, I. A. 1973. The biosystematics of the genus Thelypodium (Cruciferae). Contrib. Gray Herb. 204: 3-148. 1977 2. Al-Shehbaz, I. A. 1977. Protogyny, Cruciferae. Syst. Bot. 2: 327-333. 3. A. R. Al-Mayah & I. A. Al-Shehbaz. 1977. Chromosome numbers for some Leguminosae from Iraq. Bot. Notiser 130: 437-440. 1978 4. Al-Shehbaz, I. A. 1978. Chromosome number reports, certain Cruciferae from Iraq. -

Phylogenetic Position and Generic Limits of Arabidopsis (Brassicaceae)
PHYLOGENETIC POSITION Steve L. O'Kane, Jr.2 and Ihsan A. 3 AND GENERIC LIMITS OF Al-Shehbaz ARABIDOPSIS (BRASSICACEAE) BASED ON SEQUENCES OF NUCLEAR RIBOSOMAL DNA1 ABSTRACT The primary goals of this study were to assess the generic limits and monophyly of Arabidopsis and to investigate its relationships to related taxa in the family Brassicaceae. Sequences of the internal transcribed spacer region (ITS-1 and ITS-2) of nuclear ribosomal DNA, including 5.8S rDNA, were used in maximum parsimony analyses to construct phylogenetic trees. An attempt was made to include all species currently or recently included in Arabidopsis, as well as species suggested to be close relatives. Our ®ndings show that Arabidopsis, as traditionally recognized, is polyphyletic. The genus, as recircumscribed based on our results, (1) now includes species previously placed in Cardaminopsis and Hylandra as well as three species of Arabis and (2) excludes species now placed in Crucihimalaya, Beringia, Olimar- abidopsis, Pseudoarabidopsis, and Ianhedgea. Key words: Arabidopsis, Arabis, Beringia, Brassicaceae, Crucihimalaya, ITS phylogeny, Olimarabidopsis, Pseudoar- abidopsis. Arabidopsis thaliana (L.) Heynh. was ®rst rec- netic studies and has played a major role in un- ommended as a model plant for experimental ge- derstanding the various biological processes in netics over a half century ago (Laibach, 1943). In higher plants (see references in Somerville & Mey- recent years, many biologists worldwide have fo- erowitz, 2002). The intraspeci®c phylogeny of A. cused their research on this plant. As indicated by thaliana has been examined by Vander Zwan et al. Patrusky (1991), the widespread acceptance of A. (2000). Despite the acceptance of A. -

P020110307527551165137.Pdf
CONTENT 1.MESSAGE FROM DIRECTOR …………………………………………………………………………………………………………………………………………………… 03 2.ORGANIZATION STRUCTURE …………………………………………………………………………………………………………………………………………………… 05 3.HIGHLIGHTS OF ACHIEVEMENTS …………………………………………………………………………………………………………………………………………… 06 Coexistence of Conserve and Research----“The Germplasm Bank of Wild Species ” services biodiversity protection and socio-economic development ………………………………………………………………………………………………………………………………………………… 06 The Structure, Activity and New Drug Pre-Clinical Research of Monoterpene Indole Alkaloids ………………………………………… 09 Anti-Cancer Constituents in the Herb Medicine-Shengma (Cimicifuga L) ……………………………………………………………………………… 10 Floristic Study on the Seed Plants of Yaoshan Mountain in Northeast Yunnan …………………………………………………………………… 11 Higher Fungi Resources and Chemical Composition in Alpine and Sub-alpine Regions in Southwest China ……………………… 12 Research Progress on Natural Tobacco Mosaic Virus (TMV) Inhibitors…………………………………………………………………………………… 13 Predicting Global Change through Reconstruction Research of Paleoclimate………………………………………………………………………… 14 Chemical Composition of a traditional Chinese medicine-Swertia mileensis……………………………………………………………………………… 15 Mountain Ecosystem Research has Made New Progress ………………………………………………………………………………………………………… 16 Plant Cyclic Peptide has Made Important Progress ………………………………………………………………………………………………………………… 17 Progresses in Computational Chemistry Research ………………………………………………………………………………………………………………… 18 New Progress in the Total Synthesis of Natural Products ……………………………………………………………………………………………………… -
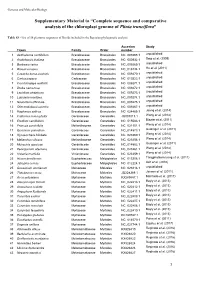
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

Downloaded from Genbank on That Full Plastid Genomes Are Not Sufficient to Reject Al- February 28, 2012
Ruhfel et al. BMC Evolutionary Biology 2014, 14:23 http://www.biomedcentral.com/1471-2148/14/23 RESEARCH ARTICLE Open Access From algae to angiosperms–inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes Brad R Ruhfel1*, Matthew A Gitzendanner2,3,4, Pamela S Soltis3,4, Douglas E Soltis2,3,4 and J Gordon Burleigh2,4 Abstract Background: Next-generation sequencing has provided a wealth of plastid genome sequence data from an increasingly diverse set of green plants (Viridiplantae). Although these data have helped resolve the phylogeny of numerous clades (e.g., green algae, angiosperms, and gymnosperms), their utility for inferring relationships across all green plants is uncertain. Viridiplantae originated 700-1500 million years ago and may comprise as many as 500,000 species. This clade represents a major source of photosynthetic carbon and contains an immense diversity of life forms, including some of the smallest and largest eukaryotes. Here we explore the limits and challenges of inferring a comprehensive green plant phylogeny from available complete or nearly complete plastid genome sequence data. Results: We assembled protein-coding sequence data for 78 genes from 360 diverse green plant taxa with complete or nearly complete plastid genome sequences available from GenBank. Phylogenetic analyses of the plastid data recovered well-supported backbone relationships and strong support for relationships that were not observed in previous analyses of major subclades within Viridiplantae. However, there also is evidence of systematic error in some analyses. In several instances we obtained strongly supported but conflicting topologies from analyses of nucleotides versus amino acid characters, and the considerable variation in GC content among lineages and within single genomes affected the phylogenetic placement of several taxa. -

Annual Report 2009
XISHUANGBANNA TROPICAL BOTANICAL GARDEN, CHINESE ACADEMY OF SCIENCES Headquarter Kunming Division Menglun, Mengla 88 Xuefu Road, Kunming Yunnan 666303, P. R. China Yunnan 650223, P. R. China Tel. + 86 691 8715460 Tel. + 86 871 5171169 Fax. + 86 691 8715070 Fax. + 86 871 5160916 www.xtbg.cas.cn Annual Report 2009 Captions for cover photos (anti-clockwise ) 1. Physiognomy of Bulong Nature Reserve; 2. Celebration of the 50th Anniversary; 3. Exhibition in Wuhan Botanical Garden; 4. Wild edible plants collection; 5. The 5th International Symposium on Zingiberaceae Xishuangbanna Tropical Botanical Garden 6. 2009 Graduation ceremony; 7. Experts’ visit to the construction site of the Chinese Academy of Sciences new research center Prepared by: FANG Chunyan HU Huabin Edited by: CHEN Jin Annual Report 2009 Xishuangbanna Tropical Botanical Garden Chinese Academy of Sciences March 31, 2010 Xishuangbanna Tropical Botanical Garden (XTBG), Chinese Academy of Sciences is a non-profit, comprehensive botanical garden involved in scientific research, plant diversity conservation and public science education, affiliated directly to the Chinese Academy of Sciences. XTBG’s vision: Financial Review Desirable base for plant diversity conservation and ecological studies. Noah’s Ark for tropical plants. XTBG’s mission: Promote science development and environmental conservation through implementing scientific research on ecology and plant diversity conservation, horticultural exhibition, and public education. 2 CONTENTS th XTBG 50 Anniversary ................................................................................. -

Synergid Calcium Ion Oscillations Define a New Feature of Pollen Tube Reception
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.23.218263; this version posted July 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Synergid calcium ion oscillations define a new feature of pollen tube reception critical for blocking interspecific hybridization Nathaniel Ponvert, and Mark A. Johnson* Department of Molecular Biology, Cell Biology, and Biochemistry; Brown University, Providence, RI, 02912. [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.07.23.218263; this version posted July 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Reproductive isolation leads to the evolution of distinct new species, however, the molecular mechanisms that promote and maintain reproductive barriers are elusive. In flowering plants sperm cells are immotile and are delivered to female gametes by the pollen grain which germinates a polarized extension, the pollen tube, into floral tissue. After growing via polar extension to the female gametophyte, the pollen tube signals with receptive female cells flanking the egg in two distinct steps. If signaling is successful, the pollen tube releases sperm cells for fusion with female gametes. To better understand cell-cell recognition during reproduction, we investigated calcium ion dynamics associated with interspecific cell signaling between mating partners. -

Publications of Ihsan A
TAXA NAMED IN HONOR OF IHSAN A. AL-SHEHBAZ 1. Tribe Shehbazieae D. A. German, Turczaninowia 17(4): 22. 2014. 2. Shehbazia D. A. German, Turczaninowia 17(4): 20. 2014. 3. Shehbazia tibetica (Maxim.) D. A. German, Turczaninowia 17(4): 20. 2014. 4. Astragalus shehbazii Zarre & Podlech, Feddes Repert. 116: 70. 2005. 5. Bornmuellerantha alshehbaziana Dönmez & Mutlu, Novon 20: 265. 2010. 6. Centaurea shahbazii Ranjbar & Negaresh, Edinb. J. Bot. 71: 1. 2014. 7. Draba alshehbazii Klimeš & D. A. German, Bot. J. Linn. Soc. 158: 750. 2008. 8. Euphorbia shehbaziana S.Y.Hama & S.A.Ahmad, Harvard Pap. Bot. 25: 73. 2020. 9. Ferula shehbaziana S. A. Ahmad, Harvard Pap. Bot. 18: 99. 2013. 10. Matthiola shehbazii Ranjbar & Karami, Nordic J. Bot. 32: 714. 2013. 11. Plocama alshehbazii F. O. Khass., D. Khamr., U. Khuzh. & Achilova, Stapfia 101: 25. 2014. 12. Alshehbazia Salariato & Zuloaga, Kew Bulletin 70: 5. 2015 13. Alshehbzia hauthalii (Gilg & Muschl.) Salariato & Zuloaga, Kew Bulletin 70: 5. 2015 14. Ihsanalshehbazia Tahir Ali & Thines, Taxon 65: 93. 2016. 15. Ihsanalshehbazia granatensis (Boiss. & Reuter) Tahir Ali & Thines, Taxon 65. 93. 2016. 16. Aubrieta alshehbazii Dönmez, Ugurlu & M.A.Koch, Phytotaxa 299. 104. 2017. 17. Silene shehbazii S.A.Ahmad, Novon 25: 131. 2017. PUBLICATIONS OF IHSAN A. AL-SHEHBAZ 1973 1. Al-Shehbaz, I. A. 1973. The biosystematics of the genus Thelypodium (Cruciferae). Contrib. Gray Herb. 204: 3-148. 1977 2. Al-Shehbaz, I. A. 1977. Protogyny, Cruciferae. Syst. Bot. 2: 327-333. 3. A. R. Al-Mayah & I. A. Al-Shehbaz. 1977. Chromosome numbers for some Leguminosae from Iraq. -

The Complete Plastid Genome of the Holoparasite Cytinus Hypocistis (Cytinaceae)
Annals of Botany 118: 885–896, 2016 doi:10.1093/aob/mcw135, available online at www.aob.oxfordjournals.org Understanding the evolution of holoparasitic plants: the complete plastid genome of the holoparasite Cytinus hypocistis (Cytinaceae) Cristina Roquet1,2,*, Eric Coissac1,2, Corinne Cruaud3, Martı Boleda1,2, Fre´de´ric Boyer1,2, Adriana Alberti3, Ludovic Gielly1,2, Pierre Taberlet1,2, Wilfried Thuiller1,2,Je´re´mie Van Es4 and Se´bastien Lavergne1,2 1Laboratoire d’Ecologie Alpine, Universite´Grenoble Alpes, BP 53, FR-38000 Grenoble, France, 2Laboratoire d’Ecologie Alpine, CNRS, BP 53, FR-38000 Grenoble, France, 3CEA-Institut de Ge´nomique, Genoscope, Centre National de Se´quenc¸age, FR-91057 Evry Cedex, France and 4Conservatoire Botanique National Alpin, Domaine de Charance, FR-05000 Gap, France *For correspondence. E-mail [email protected] Received: 2 March 2016 Returned for revision: 9 April 2016 Accepted: 12 May 2016 Published electronically: 21 July 2016 Downloaded from Background and Aims Plant plastid genomes are highly conserved in size, gene content and structure; however, parasitic plants are a noticeable exception to this evolutionary stability. Although the evolution of parasites could help to better understand plastome evolution in general, complete plastomes of parasites have been sequenced only for some lineages so far. Here we contribute to filling this gap by providing and analysing the complete plastome se- quence of Cytinus hypocistis, the first parasite sequenced for Malvales and a species suspected to have an extremely http://aob.oxfordjournals.org/ small genome. Methods We sequenced and assembled de novo the plastid genome of Cytinus hypocistis using a shotgun ap- proach on genomic DNA. -

Mandakova TPC2010, Suppl Mat.Pdf
Supplemental Data. Mandáková et al. (2010). Plant Cell 10.1105/tpc.110.074526 Supplemental Figure 1. A Three-Way Comparison of the Relative Position of Corresponding Synteny Blocks of Stenopetalum nutans (SN), S. lineare (SL) and Ballantinia antipoda (BA) Relative to the Reference Ancestral Crucifer Karyotype (ACK). In the main panel of the image, each of the three modern karyotypes is presented in a radial layout. Within each of the three karyotypes, ideograms are ordered and oriented in the outward direction (corresponding to the same counter-clockwise scale progression of Figure 3). Each line connects a pair of genomic positions on two different modern genomes that are syntenically related to the same genomic block in the ACK. For example, the light-blue line at the top of the figure between SL and SN corresponds to synteny with the genomic block U2 on AK7. Each of the eight small panels shows synteny relationships between the three modern genomes for a specific ancestral chromosome (AK1-8). Supplemental Data. Mandáková et al. (2010). Plant Cell 10.1105/tpc.110.074526 Supplemental Figure 2. The Unique Rearrangement of the AK8(#1)-like Homoeologue Shared by All Analyzed Species. This rearrangement was mediated by two subsequent paracentric inversions involving two thirds of genomic block W1 and a major part of X1. In S. lineare, block V1 underwent a secondary translocation to another chromosome. The rearrangement not shown for Arabidella eremigena and Blennodia canescens. Supplemental Data. Mandáková et al. (2010). Plant Cell 10.1105/tpc.110.074526 Supplemental Figure 3. Phylogeny of the Malate Synthase (MS) (TrN + Γ + I) Showing the Position of Sequences from the Australian Species (in Bold) in the Context of Other Brassicaceae Taxa. -

BRASSICACEAE (CRUCIFERAE) 十字花科 Shi Zi Hua Ke Zhou Taiyan (周太炎 Cheo Tai-Yien)1, Lu Lianli (陆莲立 Lou Lian-Li)1, Yang Guang (杨光)1; Ihsan A
Flora of China 8: 1–193. 2001. BRASSICACEAE (CRUCIFERAE) 十字花科 shi zi hua ke Zhou Taiyan (周太炎 Cheo Tai-yien)1, Lu Lianli (陆莲立 Lou Lian-li)1, Yang Guang (杨光)1; Ihsan A. Al-Shehbaz2 Herbs annual, biennial, or perennial, sometimes subshrubs or shrubs, with a pungent, watery juice. Eglandular trichomes unicellular, simple, stalked or sessile, 2- to many forked, stellate, dendritic, or malpighiaceous (medifixed, bifid, appressed), rarely peltate and scalelike; glandular trichomes multicellular, with uniseriate or multiseriate stalk. Stems erect, ascending, or prostrate, sometimes absent. Leaves exstipulate, simple, entire or variously pinnately dissected, rarely trifoliolate or pinnately, palmately, or bipinnately compound; basal leaf rosette present or absent; cauline leaves almost always alternate, rarely opposite or whorled, petiolate or sessile, sometimes absent. Inflorescence bracteate or ebracteate racemes, corymbs, or panicles, sometimes flowers solitary on long pedicels originating from axils of rosette leaves. Flowers hypogynous, mostly actinomorphic. Sepals 4, in 2 decussate pairs, free or rarely united, not saccate or lateral (inner) pair saccate. Petals 4, alternate with sepals, arranged in the form of a cross (cruciform; hence the earlier family name Cruciferae), rarely rudimentary or absent. Stamens 6, in 2 whorls, tetradynamous (lateral (outer) pair shorter than median (inner) 2 pairs), rarely equal or in 3 pairs of unequal length, sometimes stamens 2 or 4, very rarely 8–24; filaments slender, winged, or appendaged, median pairs free or rarely united; anthers dithecal, dehiscing by longitudinal slits. Pollen grains 3-colpate, trinucleate. Nectar glands receptacular, highly diversified in number, shape, size, and disposition around base of filaments, always present opposite bases of lateral filaments, median glands present or absent. -

Supporting Information
Supporting Information Beilstein et al. 10.1073/pnas.0909766107 SI Materials and Methods at the deepest node of the tree and 20.8 Mya for the most- Evaluation of Potential Fossil Calibrations. We searched the pa- derived node calibration (Table S3). leobotanical literature and identified 32 fossils assigned to All other fossils were used as minimum age constraints in r8s. Brassicales (Table S1). Only six (Akania americana, Akania pa- We calibrated two different nodes with the Akania fossils; the tagonica, Akania sp., Capparidoxylon holleisii, Dressiantha bi- Akania americana/A. patagonica fossils are from a more recent carpellata, Thlaspi primaevum) could be placed confidently in deposit than Akania sp. (Table S1), and thus we used the Brassicales. A fossil was considered acceptable for use as an age younger date for these fossils to constrain the divergence of constraint only if its record included a clear citation with pho- Akania bidwillii and Bretschneidera sinensis. Akania sp. was used tographic evidence or accurate reproduction, fossil collection to constrain the node defined by A. bidwilli and Tropaeolum number, and morphological characters that support the pro- majus, which is deeper in the tree than the split constrained by A. posed placement. americana/A. patagonica. This strategy allowed us to use all Akania fossils as calibrations in the ndhF and combined analyses. Ultrametric Tree and Divergence Date Estimation. To calculate di- We lacked PHYA data for B. sinensis, precluding the use of vergence dates for Brassicales, we first inferred trees from plastid A. americana/A. patagonica as a calibration in PHYA analyses. ndhF and the nuclear locus phytochrome A (PHYA)datasepa- Morphological analysis of Capparidoxylon holleisii using Inside rately and then from combined ndhFandPHYA data (Table S2).