ROLLING CONTACT ORTHOPAEDIC JOINT DESIGN by Alexander Henry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synovial Joints Permit Movements of the Skeleton
8 Joints Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes 8.1 Contrast the major categories of joints, and explain the relationship between structure and function for each category. 8.2 Describe the basic structure of a synovial joint, and describe common accessory structures and their functions. 8.3 Describe how the anatomical and functional properties of synovial joints permit movements of the skeleton. © 2018 Pearson Education, Inc. Section 1: Joint Structure and Movement Learning Outcomes (continued) 8.4 Describe flexion/extension, abduction/ adduction, and circumduction movements of the skeleton. 8.5 Describe rotational and special movements of the skeleton. © 2018 Pearson Education, Inc. Module 8.1: Joints are classified according to structure and movement Joints, or articulations . Locations where two or more bones meet . Only points at which movements of bones can occur • Joints allow mobility while preserving bone strength • Amount of movement allowed is determined by anatomical structure . Categorized • Functionally by amount of motion allowed, or range of motion (ROM) • Structurally by anatomical organization © 2018 Pearson Education, Inc. Module 8.1: Joint classification Functional classification of joints . Synarthrosis (syn-, together + arthrosis, joint) • No movement allowed • Extremely strong . Amphiarthrosis (amphi-, on both sides) • Little movement allowed (more than synarthrosis) • Much stronger than diarthrosis • Articulating bones connected by collagen fibers or cartilage . Diarthrosis (dia-, through) • Freely movable © 2018 Pearson Education, Inc. Module 8.1: Joint classification Structural classification of joints . Fibrous • Suture (sutura, a sewing together) – Synarthrotic joint connected by dense fibrous connective tissue – Located between bones of the skull • Gomphosis (gomphos, bolt) – Synarthrotic joint binding teeth to bony sockets in maxillae and mandible © 2018 Pearson Education, Inc. -

Traumatologia Hiztegia
Traumatologia HIZTEGIA KULTURA ETA HIZKUNTZA POLITIKA SAILA DEPARTAMENTO DE CULTURA Y POLÍTICA LINGÜÍSTICA Vitoria-Gasteiz, 2017 Lan honen bibliografia-erregistroa Eusko Jaurlaritzaren Bibliotekak sarearen katalogoan aurki daiteke: http://www.bibliotekak.euskadi.eus/WebOpac Argitaraldia: 1.a, 2017ko xxxx Ale-kopurua: 1.500 ale © argitaraldi honena: Euskal Autonomia Erkidegoko Administrazio Orokorra Argitaratzailea: Eusko Jaurlaritzaren Argitalpen Zerbitzu Nagusia Servicio Central de Publicaciones del Gobierno Vasco Donostia-San Sebastián, 1 - 01010 Vitoria-Gasteiz Internet: http://www.euskara.euskadi.eus/euskalterm Azala: Concetta Probanza Inprimaketa: XXXXXXXXXXXXXXXXXXX ISBN: XXXXXXXXXXXXXX Lege gordailua: XXXXXXXXX HITZAURREA Beste edozein hizkuntza bezalaxe, euskara ere berritzen eta modernizatzen doa egunetik egunera, bizirik dagoen seinale. Bide horretan ezinbestekoa da euskararen aberastasun lexikoa elikatzea, zaintzea eta sustatzea, bai hizkuntza bera normalizatzeko, bai erabiltzaileen premietara egokitzeko. Hain zuzen ere, helburu horiek bete nahian ikusi du argia eskuartean duzun hiztegi honek, orrialde hauetan jorratzen den eremuko erabiltzaile eta hiztunek lanabes erabilgarria izan dezaten beren egunerokoan. Hiztegi honetan aurkituko duzun terminologia Euskararen Aholku Batzordearen Terminologia Batzordeak gomendatutakoa da. Batzordeari dagokio, besteak beste, terminologia-alorrean dauden lehentasunak finkatzea, lan-proposamenak egitea, terminologia- lanerako irizpideak ezartzea, ponderazio-markak finkatuta termino lehiakideen -

1. Synarthrosis - Immovable
jAnatomy Lecture Notes Chapter 9 I. classification A. by function - 1. synarthrosis - immovable 2. amphiarthrosis - slightly movable 3. diarthrosis - freely movable B. by structure - material attaching bones together 1. fibrous -.dense c.t., no joint cavity a. suture - very thin, short fibers synostosis - ossification of fibrous c.t. in a suture joint b. syndesmosis - ligament (the longer the fibers the more movement is possible) c. gomphosis - periodontal ligament holds teeth in alveoli 2. cartilaginous - cartilage, no joint cavity a. synchondrosis - hyaline cartilage b. symphysis - fibrocartilage 3. synovial - joint capsule and ligaments II. structure of a synovial joint A. bone and articular cartilage (hyaline) • articular cartilage cushions bone ends by absorbing compression stress Strong/Fall 2008 page 1 jAnatomy Lecture Notes Chapter 9 B. articular capsule 1. fibrous capsule - dense irregular c.t.; holds bones together 2. synovial membrane - areolar c.t. with some simple squamous e.; makes synovial fluid C. joint cavity and synovial fluid 1. synovial fluid consists of: • fluid that is filtered from capillaries in the synovial membrane • glycoprotein molecules that are made by fibroblasts in the synovial membrane 2. fluid lubricates surface of bones inside joint capsule D. ligaments - made of dense fibrous c.t.; strengthen joint • capsular • extracapsular • intracapsular E. articular disc / meniscus - made of fibrocartilage; improves fit between articulating bones F. bursae - membrane sac enclosing synovial fluid found around some joints; cushion ligaments, muscles, tendons, skin, bones G. tendon sheath - elongated bursa that wraps around a tendon Strong/Fall 2008 page 2 jAnatomy Lecture Notes Chapter 9 III. movements at joints flexion extension abduction adduction circumduction rotation inversion eversion protraction retraction supination pronation elevation depression opposition dorsiflexion plantar flexion gliding Strong/Fall 2008 page 3 jAnatomy Lecture Notes Chapter 9 IV. -

Gen Anat-Joints
JOINTS Joint is a junction between two or more bones Classification •Functional Based on the range and type of movement they permit •Structural On the basis of their anatomic structure Functional Classification • Synarthrosis No movement e.g. Fibrous joint • Amphiarthrosis Slight movement e.g. Cartilagenous joint • Diarthrosis Movement present Cavity present Also called as Synovial joint eg.shoulder joint Structural Classification Based on type of connective tissue binding the two adjacent articulating bones Presence or absence of synovial cavity in between the articulating bone • Fibrous • Cartilagenous • Synovial Fibrous Joint Bones are connected to each other by fibrous (connective ) tissue No movement No synovial cavity • Suture • Syndesmosis • Gomphosis Sutural Joints • A thin layer of dens fibrous tissue binds the adjacent bones • These appear between the bones which ossify in membrane • Present between the bones of skull e.g . coronal suture, sagittal suture • Schindylesis: – rigid bone fits in to a groove on a neighbouring bone e.g. Vomer and sphenoid Gomphosis • Peg and socket variety • Cone shaped root of tooth fits in to a socket of jaw • Immovable • Root is attached to the socket by fibrous tissue (periodontal ligament). Syndesmosis • Bony surfaces are bound together by interosseous ligament or membrane • Membrane permits slight movement • Functionally classified as amphiarthrosis e.g. inferior tibiofibular joint Cartilaginous joint • Bones are held together by cartilage • Absence of synovial cavity . Synchondrosis . Symphysis Synchondrosis • Primary cartilaginous joint • Connecting material between two bones is hyaline cartilage • Temporary joint • Immovable joint • After a certain age cartilage is replaced by bone (synostosis) • e.g. Epiphyseal plate connecting epiphysis and diphysis of a long bone, joint between basi-occiput and basi-sphenoid Symphysis • Secondary cartilaginous joint (fibrocartilaginous joint) • Permanent joint • Occur in median plane of the body • Slightly movable • e.g. -

Articulations: Synarthrosis and Amphiarthrosis
Articulations: Synarthrosis and Amphiarthrosis It's common to think of the skeletal system as being made up of only bones, and performing only the function of supporting the body. However, the skeletal system also contains other structures, and performs a variety of functions for the body. While the bones of the skeletal system are fascinating, it is our ability to move segments of the skeleton in relation to one another that allows us to move around. Each connection of bones is called an articulationor a joint. Articulations are classified based on material at the joint and the movement allowed at the joint. Synarthrosis Articulations Immovable articulations are synarthrosis articulations ("syn" means together and "arthrosis" means joint); immovable articulations sounds like a contradiction, but all regions where bones come together are called articulations, so there are articulations that don't move, including in the skull, where bones have fused, and where your teeth meet your jaw. These synarthroses are joined with fibrous connective tissue. Some synarthroses are formed by hyaline cartilage, such as the articulation between the first rib and the sternum (via costal cartilage). This immoveable joint helps stabilize the shoulder girdle and the cartilage can ossify in adults with age. The epiphyseal plate or “growth plate” at the end of long bones is also a synarthrosis until hyaline cartilage ossification is completed around the time of puberty. Amphiarthrosis Articulations There are some articulations which have limited motion called amphiarthrosis articulations. They are held in place with fibrocartilage or fibrous connective tissue. The anterior pelvic girdle joint between pubic bones (pubic symphysis) and the intervertebral joints of the spinal column (discs) are examples of cartilaginous amphiarthroses. -

Neurofibromatosis Type I
Synovial Diseases in Children A. Carl Merrow, MD Corning Benton Chair for Radiology Education Department of Radiology Cincinnati Children’s Hospital Medical Center Disclosures • Pediatrics Lead Author for Amirsys-Elsevier – Royalties/Fees • Lecture heavily weighted for MRI Overview • Anatomy • Imaging: Techniques and value • Specific diseases – Etiology – Distribution of joints – Acute & chronic manifestations – Useful imaging clues Anatomy: Types of Joints • Synarthroses (immovable) • Amphiarthrosis (slightly movable) • Diarthrosis (freely movable) Anatomy: Diarthrodial Joints • Cartilage • Capsule – Ligaments – Fibrous tissue • Synovium – Lines capsule, not cartilage – Invests intra-articular ligaments & tendons Anatomy: Synovium • Two layers – Intima • Synovial lining cells (synoviocytes, SLCs) • Discontinuous layer 1-3 cells thick • Incomplete basement membrane – Subintima • Loose connective tissue • Merges with fibrous capsule Anatomy: Synovium • Purposes – Fluid formation – Nutrition of cartilage – Movement Synovium also found… • Bursae • Tendon sheaths • Synovial cysts • NOT synovial sarcoma Joint pathophysiology • Variety of processes may incite synovitis • Single vs. recurrent event destructive cycle • Long term effects – Bone – Articular cartilage – Growing epiphysis Imaging: Why? • Establish diagnosis • Localize/define extent of involvement – Within single joint – Tendon sheath/bursa – Additional unsuspected joints • Pick up complications early • Optimize management General imaging: Radiographs • Pros – Baseline – Exclude other -

4 Anat 35 Articulations
Human Anatomy Unit 1 ARTICULATIONS In Anatomy Today Classification of Joints • Criteria – How bones are joined together – Degree of mobility • Minimum components – 2 articulating bones – Intervening tissue • Fibrous CT or cartilage • Categories – Synarthroses – no movement – Amphiarthrosis – slight movement – Diarthrosis – freely movable Synarthrosis • Immovable articulation • Types – Sutures – Schindylesis – Gomphosis – Synchondrosis Synarthrosis Sutures • Found only in skull • Immovable articulation • Flat bones joined by thin layer of fibrous CT • Types – Serrate – Squamous (lap) – Plane Synarthrosis Sutures • Serrate • Serrated edges of bone interlock • Two portions of frontal bones • Squamous (lap) • Overlapping beveled margins forms smooth line • Temporal and parietal bones • Plane • Joint formed by straight, nonoverlapping edges • Palatine process of maxillae Synarthrosis Schindylesis • Immovable articulation • Thin plate of bone into cleft or fissure in a separation of the laminae in another bone • Articulation of sphenoid bone and perpendicular plate of ethmoid bone with vomer Synarthrosis Gomphosis • Immovable articulation • Conical process into a socket • Articulation of teeth with alveoli of maxillary bone • Periodontal ligament = fibrous CT Synarthrosis Synchondrosis • Cartilagenous joints – Ribs joined to sternum by hyaline cartilage • Synostoses – When joint ossifies – Epiphyseal plate becomes epiphyseal line Amphiarthrosis • Slightly moveable articulation • Articulating bones connected in one of two ways: – By broad flattened -

FIPAT-TA2-Part-2.Pdf
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART II Contents: Systemata musculoskeletalia Musculoskeletal systems Caput II: Ossa Chapter 2: Bones Caput III: Juncturae Chapter 3: Joints Caput IV: Systema musculare Chapter 4: Muscular system Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput II: OSSA Chapter 2: BONES Latin term Latin synonym UK English US English English synonym Other 351 Systemata Musculoskeletal Musculoskeletal musculoskeletalia systems systems -

Synovial Joints
Chapter 9 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • Joints link the bones of the skeletal system, permit effective movement, and protect the softer organs • Joint anatomy and movements will provide a foundation for the study of muscle actions 9-2 Joints and Their Classification • Expected Learning Outcomes – Explain what joints are, how they are named, and what functions they serve. – Name and describe the four major classes of joints. – Describe the three types of fibrous joints and give an example of each. – Distinguish between the three types of sutures. – Describe the two types of cartilaginous joints and give an example of each. – Name some joints that become synostoses as they age. 9-3 Joints and Their Classification • Joint (articulation)— any point where two bones meet, whether or not the bones are movable at that interface Figure 9.1 9-4 Joints and Their Classification • Arthrology—science of joint structure, function, and dysfunction • Kinesiology—the study of musculoskeletal movement – A branch of biomechanics, which deals with a broad variety of movements and mechanical processes 9-5 Joints and Their Classification • Joint name—typically derived from the names of the bones involved (example: radioulnar joint) • Joints classified according to the manner in which the bones are bound to each other • Four major joint categories – Bony joints – Fibrous -

Vocabulario De Medicina
Vocabulario de Medicina (galego-español-inglés-portugués) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN DE VOCABULARIOS TEMÁTICOS N.º 5 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Medicina (galego-español-inglés-portugués) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de medicina : (galego-español-inglés-portugués) /coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores María Casas García ... [et al.]. — Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. — 851 p. ; 21 cm. — (Vocabularios temáticos ; 5). — D.L.C 3806-2008. — ISBN 978-84-9887-028-2 1. Medicina-Diccionarios. 2. Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Rodríguez Río, Xusto A., coord. II.Casas García María. III.Universidade de Santiago de Compostela, Servizo de Normalización Lingüística, coord. IV. Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 61(038)=699=60=20=690 © Universidade de Santiago de Compostela, 2008 Coordinador: Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res: María Casas García (Área de Medicina Familiar e Comunitaria. Unidade Docente de Pontevedra. Centro de Saúde de Bueu) Sonia Miguélez Ferreiro (Área de Medicina Familiar y Comunitaria. Unidad Docente de Segovia. Centro de Salud Segovia 1) Carolina Pena Álvarez (Área de Oncoloxía Médica. Complexo Hospitalario de Pontevedra) Iria Pereira Fernández (Escola Universitària d’Infermeria. Universitat de Barcelona) Adriana Rubín Barrenechea (Hospital Amato Lusitano. Castelo Branco. Portugal) Sabela Sánchez Trigo (Área de Medicina Interna. Complexo Hospitalario Arquitecto Marcide - Nóvoa Santos. Ferrol) Xoana María Vázquez Vicente (Servei d’Aparell Digestiu. -

Cartilaginous Joints*
OpenStax-CNX module: m46381 1 Cartilaginous Joints* OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 Abstract By the end of this section, you will be able to: • Describe the structural features of cartilaginous joints • Distinguish between a synchondrosis and symphysis • Give an example of each type of cartilaginous joint As the name indicates, at a cartilaginous joint, the adjacent bones are united by cartilage, a tough but exible type of connective tissue. These types of joints lack a joint cavity and involve bones that are joined together by either hyaline cartilage or brocartilage (Figure 1 (Cartiliginous Joints )). There are two types of cartilaginous joints. A synchondrosis is a cartilaginous joint where the bones are joined by hyaline cartilage. Also classied as a synchondrosis are places where bone is united to a cartilage structure, such as between the anterior end of a rib and the costal cartilage of the thoracic cage. The second type of cartilaginous joint is a symphysis, where the bones are joined by brocartilage. *Version 1.3: Jun 4, 2013 10:16 am -0500 http://creativecommons.org/licenses/by/3.0/ http://cnx.org/content/m46381/1.3/ OpenStax-CNX module: m46381 2 Cartiliginous Joints Figure 1: At cartilaginous joints, bones are united by hyaline cartilage to form a synchondrosis or by brocartilage to form a symphysis. (a) The hyaline cartilage of the epiphyseal plate (growth plate) forms a synchondrosis that unites the shaft (diaphysis) and end (epiphysis) of a long bone and allows the bone to grow in length. -
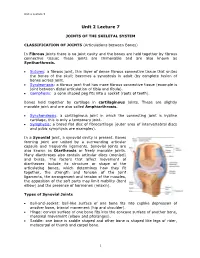
Joints of the Skeletal System
Unit 2 Lecture 5 Unit 2 Lecture 7 JOINTS OF THE SKELETAL SYSTEM CLASSIFICATION OF JOINTS (Articulations between Bones) In Fibrous joints there is no joint cavity and the bones are held together by fibrous connective tissue; these joints are Immovable and are also known as Syntharthrosis. Sutures: a fibrous joint, thin layer of dense fibrous connective tissue that unites the bones of the skull; becomes a synostosis in adult (by complete fusion of bones across joint. Syndesmosis: a fibrous joint that has more fibrous connective tissue (example is joint between distal articulation of tibia and fibula). Gomphosis: a cone shaped peg fits into a socket (roots of teeth). Bones held together by cartilage in cartilaginous joints. These are slightly movable joint and are also called Amphiarthrosis. Synchondrosis: a cartilaginous joint in which the connecting joint is hyaline cartilage, this is only a temporary joint. Symphysis: a broad flat disc of fibrocartilage (outer area of intervertebral discs and pubic symphysis are examples). In a Synovial joint, a synovial cavity is present. Bones forming joint are united by a surrounding articular capsule and frequently ligaments. Synovial joints are also known as Diarthrosis or freely movable joints. Many diarthroses also contain articular discs (menisci) and bursa. The factors that affect movement of diarthroses include its structure or shape of the articulating bones, which determines how they fit together, the strength and tension of the joint ligaments, the arrangement and tension of the muscles, the apposition of the soft parts may limit mobility (bent elbow) and the presence of hormones (relaxin). Types of Synovial Joints Ball-and-socket: ball-like surface of one bone fits into cuplike depression of another bone, triaxial movement (hip and shoulder).