Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HUNTIA a Journal of Botanical History
HUNTIA A Journal of Botanical History VOLUME 15 NUMBER 2 2015 Hunt Institute for Botanical Documentation Carnegie Mellon University Pittsburgh The Hunt Institute for Botanical Documentation, a research division of Carnegie Mellon University, specializes in the history of botany and all aspects of plant science and serves the international scientific community through research and documentation. To this end, the Institute acquires and maintains authoritative collections of books, plant images, manuscripts, portraits and data files, and provides publications and other modes of information service. The Institute meets the reference needs of botanists, biologists, historians, conservationists, librarians, bibliographers and the public at large, especially those concerned with any aspect of the North American flora. Huntia publishes articles on all aspects of the history of botany, including exploration, art, literature, biography, iconography and bibliography. The journal is published irregularly in one or more numbers per volume of approximately 200 pages by the Hunt Institute for Botanical Documentation. External contributions to Huntia are welcomed. Page charges have been eliminated. All manuscripts are subject to external peer review. Before submitting manuscripts for consideration, please review the “Guidelines for Contributors” on our Web site. Direct editorial correspondence to the Editor. Send books for announcement or review to the Book Reviews and Announcements Editor. Subscription rates per volume for 2015 (includes shipping): U.S. $65.00; international $75.00. Send orders for subscriptions and back issues to the Institute. All issues are available as PDFs on our Web site, with the current issue added when that volume is completed. Hunt Institute Associates may elect to receive Huntia as a benefit of membership; contact the Institute for more information. -

Genetic and Biochemical Mechanisms of Pollen Wall Development
Review Genetic and Biochemical Mechanisms of Pollen Wall Development 1 1 1 1,2 Jianxin Shi, Meihua Cui, Li Yang, Yu-Jin Kim, and 1,3, Dabing Zhang * The pollen wall is a specialized extracellular cell wall matrix that surrounds male Trends gametophytes and plays an essential role in plant reproduction. Uncovering the Pollen wall development exhibits con- fi mechanisms that control the synthesis and polymerization of the precursors of served and diversi ed features. pollen wall components has been a major research focus in plant biology. We Genes associated with pollen wall devel- review current knowledge on the genetic and biochemical mechanisms under- opment are coordinately regulated. lying pollen wall development in eudicot model Arabidopsis thaliana and mono- The synthesis of exine and anther cutin cot model rice (Oryza sativa), focusing on the genes involved in the biosynthesis, may share common pathways in rice. transport, and assembly of various precursors of pollen wall components. The conserved and divergent aspects of the genes involved as well as their regula- tion are addressed. Current challenges and future perspectives are also highlighted. Pollen Wall Development The pollen wall is the complex multiple-layer outer surface of pollen. It is essential for plant reproduction because of its role in rendering male gametophytes resistant to various biotic and abiotic stresses, as well as its function in male–female interaction, fertilization, and seed production [1]. The underlying genetic, molecular, and biochemical mechanisms of pollen wall development have long defied unraveling, but this is changing fast. Several excellent reviews have summarized the genes and enzymes associated with the biosynthesis and transport of the lipidic and phenolic precursors necessary for the formation of the outer pollen wall named exine 1 Joint International Research – [1 4] (see Glossary). -

Atlas of Pollen and Plants Used by Bees
AtlasAtlas ofof pollenpollen andand plantsplants usedused byby beesbees Cláudia Inês da Silva Jefferson Nunes Radaeski Mariana Victorino Nicolosi Arena Soraia Girardi Bauermann (organizadores) Atlas of pollen and plants used by bees Cláudia Inês da Silva Jefferson Nunes Radaeski Mariana Victorino Nicolosi Arena Soraia Girardi Bauermann (orgs.) Atlas of pollen and plants used by bees 1st Edition Rio Claro-SP 2020 'DGRV,QWHUQDFLRQDLVGH&DWDORJD©¥RQD3XEOLFD©¥R &,3 /XPRV$VVHVVRULD(GLWRULDO %LEOLRWHF£ULD3ULVFLOD3HQD0DFKDGR&5% $$WODVRISROOHQDQGSODQWVXVHGE\EHHV>UHFXUVR HOHWU¶QLFR@RUJV&O£XGLD,Q¬VGD6LOYD>HW DO@——HG——5LR&ODUR&,6(22 'DGRVHOHWU¶QLFRV SGI ,QFOXLELEOLRJUDILD ,6%12 3DOLQRORJLD&DW£ORJRV$EHOKDV3µOHQ– 0RUIRORJLD(FRORJLD,6LOYD&O£XGLD,Q¬VGD,, 5DGDHVNL-HIIHUVRQ1XQHV,,,$UHQD0DULDQD9LFWRULQR 1LFRORVL,9%DXHUPDQQ6RUDLD*LUDUGL9&RQVXOWRULD ,QWHOLJHQWHHP6HUYL©RV(FRVVLVWHPLFRV &,6( 9,7¯WXOR &'' Las comunidades vegetales son componentes principales de los ecosistemas terrestres de las cuales dependen numerosos grupos de organismos para su supervi- vencia. Entre ellos, las abejas constituyen un eslabón esencial en la polinización de angiospermas que durante millones de años desarrollaron estrategias cada vez más específicas para atraerlas. De esta forma se establece una relación muy fuerte entre am- bos, planta-polinizador, y cuanto mayor es la especialización, tal como sucede en un gran número de especies de orquídeas y cactáceas entre otros grupos, ésta se torna más vulnerable ante cambios ambientales naturales o producidos por el hombre. De esta forma, el estudio de este tipo de interacciones resulta cada vez más importante en vista del incremento de áreas perturbadas o modificadas de manera antrópica en las cuales la fauna y flora queda expuesta a adaptarse a las nuevas condiciones o desaparecer. -

Characterization of Flowering Time and Pollen Production in Jojoba (Simmondsia Chinensis) Towards a Strategy for the Selection of Elite Male Genotypes
agronomy Brief Report Characterization of Flowering Time and Pollen Production in Jojoba (Simmondsia chinensis) towards a Strategy for the Selection of Elite Male Genotypes Noemi Tel Zur 1,* , Ronen Rothschild 2, Udi Zurgil 1 and Yiftach Vaknin 3 1 French Associates Institute for Agriculture and Biotechnology of Drylands, The Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Sede Boqer Campus, Beersheba 84990000, Israel; [email protected] 2 Jojoba Israel Ltd., Kibbutz Hatzerim 8542000, Israel; [email protected] 3 Institute of Plant Sciences, Agricultural Research Organization (ARO), Volcani Center, Rishon LeZion 7505101, Israel; [email protected] * Correspondence: [email protected] Received: 1 April 2020; Accepted: 13 April 2020; Published: 22 April 2020 Abstract: The seeds of the dioecious shrub jojoba (Simmondsia chinensis (Link) Schneider) yield a liquid wax that is in high demand for the cosmetics industry. While elite female cultivars of this species are currently clonally propagated, male plants are grown from seed, resulting in large variations in both the flowering period and the pollen viability, and hence large variation in yields. We characterized the existing male plant material in a local plantation as a platform for future selection of elite male cultivars that would produce sufficient amounts of viable pollen throughout the extended flowering period of the female cultivars. Using as a guide the number of viable pollen grains per 1-m branch, defined here as the calculated effective pollen productivity (EPP), we identified plants with an elevated EPP that flower concurrently with the female cultivars. Keywords: dioecious; flowering time; phenological diversity; pollen viability 1. -

How Flowering Plants Discriminate Between Self and Non-Self Pollen to Prevent Inbreeding TEH-HUI KAO and ANDREW G
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 12059-12065, October 1996 Symposium Paper This paper was presented at a symposium entitled "Frontiers in Plant Biology: How Plants Communicate, " organized by Hans Kende and held at the 133rd Annual Meeting of the National Academy of Sciences on April 30, 1996. How flowering plants discriminate between self and non-self pollen to prevent inbreeding TEH-HUI KAO AND ANDREW G. MCCUBBIN Department of Biochemistry and Molecular Biology, 403 Althouse Laboratory, Pennsylvania State University, University Park, PA 16802 ABSTRACT Flowering plants have evolved various ge- heteromorphic types. In the homomorphic type, flowers of the netic mechanisms to circumvent the tendency for self- same species have the same morphological type, whereas in the fertilization created by the close proximity of male and female heteromorphic type, flowers of the same species can have two reproductive organs in a bisexual flower. One such mecha- or three different morphological types, and pollination is nism is gametophytic self-incompatibility, which allows the compatible only between flowers of different morphological female reproductive organ, the pistil, to distinguish between types (2). The homomorphic type is further classified into self pollen and non-self pollen; self pollen is rejected, whereas gametophytic and sporophytic types based on whether the non-self pollen is accepted for fertilization. The Solanaceae pollen behavior in self-incompatibility interactions is deter- family has been used as a model to study the molecular and mined by the genotype of the pollen itself (gametophytic) or biochemical basis of self/non-self-recognition and self- by the genotype of the plant from which the pollen is derived rejection. -

Radiata Pine Pollen
Radiata pine pollen New Zealand planted forest environmental facts. Radiata pine forests in New Zealand produce large clouds of pollen every spring. This pollen may concern people who suffer from allergies. Right: Pollen catkins on the end of pine branches. Left: Pollen grain section viewed under a microscope. and contain a range of organic chemicals – proteins, What is pollen lipids, carbohydrates and nucleic acids. Pollen is produced by plants as part of their reproductive • Why pine pollen is yellow. It contains compounds called cycle. Billions of pollen grains are released into the flavonoids that can be also be orange or red. Flavonoids atmosphere by the catkins, or the flowering parts, of pine protect the pollen from the sun’s UV-B radiation and trees. prevent deformities in seeds produced with the pollen. Pollen is carried by wind. When it lands on a compatible • Pine pollen volume. A mature Pinus radiata tree can female pine catkin pollination, or fertilisation, takes place. produce between 0.5 and 0.75 kilograms of pollen each The tree then produces pine cones filled with seeds. year. At a typical 400 trees per hectare this is equivalent to up to 300 kilograms per hectare per year. • Pine pollen spread. Wind spread pollen travels less than Pollen Production 700 metres but in windy areas it will travel further. In Pine pollen is produced over a few weeks in late winter and the US, pollen has been found 300 kilometres from its early spring. If you live downwind of a pine forest then it source. Pollen that enters waterways can also travel is inevitable you will get a cloud of pollen colouring the long distances. -

Chapter 12: Life Cycles: Meiosis and the Alternation of Generations
Chapter 12 Life Cycles: Meiosis and the Alternation of Generations LIFE CYCLES TRANSFER GENETIC INFORMATION Asexual Reproduction Transfers Unchanged Genetic Information through Mitosis Sexual Reproduction Produces New Information through Meiosis and Fertilization ALTERNATION BETWEEN DIPLOID AND HAPLOID GENERATIONS Plants Vary in the Details of Their Life Cycles Sexual Cycles Can Be Heterosporic or Homosporic Only One Generation Is Multicellular in Zygotic or Gametic Life Cycles The Diploid Generation Has Become Dominant over Evolutionary Time SUMMARY 1 KEY CONCEPTS 1. Life perpetuates itself through reproduction, which is the transfer of genetic information from one generation to the next. This transfer is our definition of life cycle. Reproduction can be asexual or sexual. 2. Asexual reproduction requires a cell division know as mitosis. Asexual reproduction offers many advantages over sexual reproduction, one of which is that it requires only a single parent. A significant disadvantage of asexual reproduction is the loss of genetic diversity and the likelihood of extinction when the environment changes. 3. Sexual reproduction involves the union of two cells, called gametes, which are usually produced by two different individuals. Another kind of cell division, known as meiosis, ultimately is necessary to produce gametes. 4. Every species in the kingdom Plantae has both diploid and haploid phases--that is, plants whose cells are all diploid or all haploid. These phases are called generations, and they alternate with each other over time. 5. The fossil record reveals that the most recent groups to evolve have sporic life cycles, in which the gametophyte (haploid) generation is relatively small and the sporophyte (diploid) generation is dominant in terms of size, complexity, and longevity. -
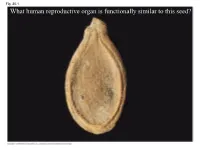
What Human Reproductive Organ Is Functionally Similar to This Seed?
Fig. 30-1 What human reproductive organ is functionally similar to this seed? Overview: Transforming the World • Seeds changed the course of plant evolution, enabling their bearers to become the dominant producers in most terrestrial ecosystems • A seed consists of an embryo and nutrients surrounded by a protective coat Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-1 What human reproductive organ is functionally similar to this seed? Seeds and pollen grains are key adaptations for life on land • In addition to seeds, the following are common to all seed plants – Reduced gametophytes – Heterospory – Ovules – Pollen Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Advantages of Reduced Gametophytes • The gametophytes of seed plants develop within the walls of spores that are retained within tissues of the parent sporophyte • Protect female gametophyte from UV and desiccation (good for the land life!) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 30-2 PLANT GROUP Mosses and other Ferns and other seedless Seed plants (gymnosperms and angiosperms) nonvascular plants vascular plants Reduced, independent Reduced (usually microscopic), dependent on surrounding (photosynthetic and Gametophyte Dominant sporophyte tissue for nutrition free-living) Sporophyte Reduced, dependent on Dominant Dominant gametophyte for nutrition Gymnosperm Angiosperm Sporophyte Microscopic female (2n) gametophytes (n) inside ovulate cone Microscopic Sporophyte -

Biol 211 (2) Chapter 31 October 9Th Lecture
S.I. Biol 211 Biology 211 (2) Week 7! Chapter 31! ! VOCABULARY! Practice: http://www.superteachertools.us/speedmatch/speedmatch.php? gamefile=4106#.VhqUYGRVhBc ! Alternation of Angiosperm: A Antheridia: The Archegonia: The generations: A life cycle flowering vascular sperm producing egg-producing involving alternation of a plant that produces structure in most structure in most multicellular haploid seed within mature land plants except land plants except ovaries (fruits). The angiosperms angiosperms stage (gametophyte) with angiosperms form a a multicellular diploid single lineage stage (sporophyte). Occurs in most plants and some protists. Artificial selection: Bisexual Carpel: The female Diploid: Having two Deliberate manipulation gametophyte: One reproductive organ sets of by humans, as in animal gametophyte that in a flower, chromosomes (2n) and plant breeding, of produces both eggs contains the ovary, the genetic composition and sperm which contains of a population by ovules, which allowing only individuals contain the with desirable traits to megasporangia reproduce Double fertilization: An Endosperm: A Fruit: In Gametangia: The unusual form of triploid (3n) tissue angiosperms, a gamete-forming reproduction seen in in the seed of a mature, ripened structure found in flowering plants, in which flowering plant plant ovary, along all land plants one sperm cell fuses with an (angiosperm) that with the seeds it except angiosperms. egg to form a zygote and the serves as food for contains and Contains an other sperm cell fuses with two polar nuclei to form the the plant embryo. adjacent fused antheridium and triploid endosperm Functionally parts, often archegonium. analogous to the functions in seed yolk of an egg dispersal Gametophyte: In Gymnosperm: A Haploid: Having Heterospory: In organisms undergoing vascular plant that one set of seed plants, the alternation of makes seeds but chromosomes production of two generations, the does not produce distinct types of multicellular haploid form flowers. -

Parts of a Flower.Pub
Angiosperms FLOWERING Plants Complete flowers have stamens, a pistil, petals, and sepals. Stigma (receives the pollen during fertilization) Anther (contains pollen, the male reproductive cell) Pistil Ovary (female reproductive Filament Stamen Stamen organ) (holds the anther) Ovule (reproductive cell which will become the seed when fertilized by pollen) Stamens: Male Reproductive Organs Pistils: Female Reproductive Organs A stamen consists of an anther (which produces The pistil includes an ovary (where the ovules are pollen, the male reproductive cell) and a filament. produced; ovules are the female reproductive cells, the eggs), and a stigma (which receives the pollen during fertilization). Fertilization Pollination is often aided by insects like bees, which fly from flower to flower; as they visit flowers, they spread pollen and deposit it on the stigmas. After pollen grains have landed on the stigma, pollen tubes develop, and burrow down into the ovary, there the pollen (sperm cell) fertilizes an ovule (egg cell). After fertilization, the ovule develops into a seed. In contrast to the idealized diagram above flowers actually are quite varied in appearance. Petals come in a wide variety of shapes and sizes, some “petals” are actually leaves. (Some types of flowers have both male and female reproductive organs (as shown above), others have only male or only female reproductive organs.) Poinsettia Chrysanthemum Lily Primrose female The colorful, showy The blossom is really This lily has both male bracts are actually a cluster of small and female parts. It is modified leaves. The flowers. The showy comparable to the true flowers are outer flowers, which idealized flower yellow and held in the look like petals, are diagram above. -

Pollen Dispersion of Some Forest Trees
Pollen Dispersion Of Some Forest Trees Northeastern Forest Experiment Station Ralph W. Marquis, Director Upper Barby, Pa. Forest Service, U.S. Dept. of Agrieultun 1 ACKNOWLEDGMENTS "a Part of the study reported in this paper was done at West Lafayette, Indiana, while the author was an instructor in forestry at Purdue University; the other part in the vicinity of Philadelphia, Pa., as part of the genetics re- search of the Northeastern Forest Experiment Station. The author gratefully acknowledges the help given by staff members of both institutions in the mathematical work and in the preparation of this report. The staff of the Morris Arboretum, Uni- versity of Pennsylvania, ,assisted by providing office and laboratory facilities as well as the use of tree specimens. A special debt of thanks is due to Pro- fessor Sewall Wright, of the Department of Zoology at the University of Chicago. He helped immeasurably by providing sample calculations, checking the author's interpretation of the data, and reviewing the manuscript. Without the use of his published works and the personal interest he took in the study most of the data would have rained a mass of figures without practical application? CONTENTS Page METHOD . Pollen collection . Calculations Regression equations & neighborhood size Pollen trajectories with rate of fall & wind velocity the controlling factors Weather data . RESULTS. Eh . Ash............. Poplar . Douglas-fir . Spruce . .. Apple ... Pinyon. ". a ... True cedar . a . FACTORS THAT INFLUENCE DISPERSION DISTANCE Wind velocity & direction . Topographyoftheland . Diameter & rate of fall of pollen grain Turbulence of the atmosphere . Height of pollen source a . POLLEN DISPERSION & RACE FORMATION . SUMMARY 0 r c . -

Plant Reproduction Asexual Reproduction
Plant Reproduction Asexual Reproduction • Asexual reproduction is natural “cloning.” Parts of the plant, such as leaves or stems, produce roots and become an independent plant. Sexual Reproduction • Sexual reproduction requires fusion of male cells in the pollen grain with female cells in the ovule. Terms to know: • Haploid: having a single set of chromosomes in each cell. • Diploid: having two sets of chromosomes in each cell, one set from each parent. • Mitosis: cell division, which produces two genetically identical diploid cells. • Meiosis: reduction division, which produces four haploid reproductive cells. Terms to know: • Spore: haploid reproductive cell that leads to a gametophyte in plant alternation of generations. • Gamete: mature haploid male or female germ cell able to unite with another of the opposite sex in sexual reproduction to form a zygote. • Zygote: diploid, eukaryotic cell formed during fertilization event between two gametes, combining DNA of each gamete, containing the genetic information to form a new individual. Terms to know: • Sporophyte: diploid, multicellular stage which develops from zygote, produced when a haploid female cell is fertilized by a haploid male cell, produces haploid spores by meiosis. • Gametophyte: haploid, multicellular stage, develops from a spore by mitosis, produces haploid gametes by mitosis. Plant Life Cycle Animals vs. Plants Plant Reproduction Animal Reproduction Alternation of No alternation of Life cycle generations generations Gametes Haploid gametes Haploid gametes Spores Haploid spores N/A (no spores) Gametes made Haploid gametophyte, Diploid organism, by by by mitosis meiosis Diploid sporophyte, by Spores made by N/A (no spores) meiosis Alternation of Generations • Plants have a double life cycle with two forms: • Sporophyte • Gametophyte Non-flowering plants • Mosses, ferns, and related plants have motile, swimming sperm.