Long QT Syndrome: from Channels to Cardiac Arrhythmias
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The QT Interval: How Long Is Too Long?
heart matters The QT interval: How long is too long? By Natalie K. Cox, RN Staff Nurse • Coronary Care Unit • McLeod Regional Medical Center • Florence, S.C. What’s the QT interval and why’s it so the appearance that the QT interval is pro- important? In this article, I’ll answer these longed. However, a wide QRS complex questions plus show you how to measure represents depolarization, and LQTS is a dis- the QT interval and how to recognize the order of repolarization. Sometimes the end types of long QT syndrome (LQTS) and of a T wave isn’t clearly defi ned, which can their symptoms, causes, and treatments. make it diffi cult to get the QT measurement. Irregular rhythms also make it diffi cult to The long and short of it obtain a consistent QT interval measure- The QT interval on the ECG is measured ment. For example, atrial fi brillation makes from the beginning of the QRS complex to it extremely diffi cult to measure a QT inter- the end of the T wave (see ECG components). val because fi nding a T wave isn’t always It represents the time it takes for the ventricles of the heart to depolarize and repolarize, or to ECG components contract and relax. The QT interval is longer when First positive deflection after the the heart rate is slower and short- ECG components P or Q wave er when the heart rate is faster. So it’s necessary to calculate the corrected QT interval (QTc) using the Bazett formula: QT interval divided by the square root of the R-R interval. -

Anemia and the QT Interval in Hypertensive Patients
2084 International Journal of Collaborative Research on Internal Medicine & Public Health Anemia and the QT interval in hypertensive patients Ioana Mozos 1*, Corina Serban 2, Rodica Mihaescu 3 1 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 2 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 3 1st Department of Internal Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania * Corresponding Author ; Email: [email protected] Abstract Introduction: A prolonged ECG QT interval duration and an increased QT dispersion (QTd) are predictors of sudden cardiac death. Anemia is known as a marker of adverse outcome in cardiovascular disease. Objective: The aim was to assess the relationship between anemia and QT intervals in hypertensive patients. Method: A total of 72 hypertensive patients underwent standard 12-lead ECG. QT intervals and QT dispersions were manually measured. Complete blood count was also assessed. Result: Linear regression analysis revealed significant associations between prolonged QTc and increased QTd and anemia and macrocytosis, respectively. Multiple regression analysis revealed a significant association between red cell distribution width (RDW) >15% and prolonged heart rate corrected maximal QT interval duration (QTc) and QT interval in lead DII (QTIIc). The most sensitive and specific predictor of prolonged QTc and QTIIc was anisocytosis. Anemia was the most sensitive predictor of -

Neurotransmitter Actions
Central University of South Bihar Panchanpur, Gaya, India E-Learning Resources Department of Biotechnology NB: These materials are taken/borrowed/modified/compiled from various resources like research articles and freely available internet websites, and are meant to be used solely for the teaching purpose in a public university, and for serving the needs of specified educational programmes. Dr. Jawaid Ahsan Assistant Professor Department of Biotechnology Central University of South Bihar (CUSB) Course Code: MSBTN2003E04 Course Name: Neuroscience Neurotransmitter Actions • Excitatory Action: – A neurotransmitter that puts a neuron closer to an action potential (facilitation) or causes an action potential • Inhibitory Action: – A neurotransmitter that moves a neuron further away from an action potential • Response of neuron: – Responds according to the sum of all the neurotransmitters received at one time Neurotransmitters • Acetylcholine • Monoamines – modified amino acids • Amino acids • Neuropeptides- short chains of amino acids • Depression: – Caused by the imbalances of neurotransmitters • Many drugs imitate neurotransmitters – Ex: Prozac, zoloft, alcohol, drugs, tobacco Release of Neurotransmitters • When an action potential reaches the end of an axon, Ca+ channels in the neuron open • Causes Ca+ to rush in – Cause the synaptic vesicles to fuse with the cell membrane – Release the neurotransmitters into the synaptic cleft • After binding, neurotransmitters will either: – Be destroyed in the synaptic cleft OR – Taken back in to surrounding neurons (reuptake) Excitable cells: Definition: Refers to the ability of some cells to be electrically excited resulting in the generation of action potentials. Neurons, muscle cells (skeletal, cardiac, and smooth), and some endocrine cells (e.g., insulin- releasing pancreatic β cells) are excitable cells. -

Young Adults. Look for ST Elevation, Tall QRS Voltage, "Fishhook" Deformity at the J Point, and Prominent T Waves
EKG Abnormalities I. Early repolarization abnormality: A. A normal variant. Early repolarization is most often seen in healthy young adults. Look for ST elevation, tall QRS voltage, "fishhook" deformity at the J point, and prominent T waves. ST segment elevation is maximal in leads with tallest R waves. Note high take off of the ST segment in leads V4-6; the ST elevation in V2-3 is generally seen in most normal ECG's; the ST elevation in V2- 6 is concave upwards, another characteristic of this normal variant. Characteristics’ of early repolarization • notching or slurring of the terminal portion of the QRS wave • symmetric concordant T waves of large amplitude • relative temporal stability • most commonly presents in the precordial leads but often associated with it is less pronounced ST segment elevation in the limb leads To differentiate from anterior MI • the initial part of the ST segment is usually flat or convex upward in AMI • reciprocal ST depression may be present in AMI but not in early repolarization • ST segments in early repolarization are usually <2 mm (but have been reported up to 4 mm) To differentiate from pericarditis • the ST changes are more widespread in pericarditis • the T wave is normal in pericarditis • the ratio of the degree of ST elevation (measured using the PR segment as the baseline) to the height of the T wave is greater than 0.25 in V6 in pericarditis. 1 II. Acute Pericarditis: Stage 1 Pericarditis Changes A. Timing 1. Onset: Day 2-3 2. Duration: Up to 2 weeks B. Findings 1. -

The Relation Between Arterial Blood Pressure Variables and Ventricular Repolarization Parameters
860 International Journal of Collaborative Research on Internal Medicine & Public Health The relation between arterial blood pressure variables and ventricular repolarization parameters Ioana Mozos 1*, Corina Serban 2, Rodica Mihaescu 3 1 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania, [email protected] 2 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 3 Department of Medical Semiology, “Victor Babes” University of Medicine and Pharmacy Timisoara, Romania ABSTRACT Introduction: Ventricular arrhythmia and sudden cardiac death risk are associated with prolonged electrocardiographic (ECG) QT and Tpeak-Tend intervals. Objective: To evaluate the influence of blood pressure variables on ventricular repolarization parameters, especially QT and Tpeak-Tend intervals. Method: Two groups of patients were enrolled in the study. The firs group included 77 patients, with essential hypertension, aged 62±12 years, 40% males. The control group included 56 patients, age and sex matched, with optimal, normal and high normal blood pressure. They underwent 12-lead ECG and ventricular repolarization parameters were assessed. QT intervals: QTmax (maximal QT interval duration), QTc (heart rate corrected QTmax), QTm (mean QT interval duration in all leads), QTIIc (heart rate corrected QT interval duration in lead DII), and T wave variables: T0e (maximal T wave duration), Tpe (maximal Tpeak-Tend interval) and Ta (maximal T wave amplitude) were manually measured. Arterial blood pressure variables: systolic (SBP), diastolic (DBP), mean arterial (MAP) and pulse pressure (PP), were recorded. Result: SBP was 139±24 mmHg, DBP 86±13 mmHg, MAP 103±15 mmHg, PP 53±16 mmHg, QTmax 430±51 ms, QTc 474±48 ms and Tpe 100±26 ms in the hypertensive group. -

Review Article Management of Atrial Fibrillation in Critically Ill Patients
Hindawi Publishing Corporation Critical Care Research and Practice Volume 2014, Article ID 840615, 10 pages http://dx.doi.org/10.1155/2014/840615 Review Article Management of Atrial Fibrillation in Critically Ill Patients Mattia Arrigo, Dominique Bettex, and Alain Rudiger Cardiosurgical Intensive Care Unit, Institute of Anesthesiology, University Hospital Zurich, Raemistraße 100, 8091 Zurich, Switzerland Correspondence should be addressed to Alain Rudiger; [email protected] Received 29 September 2013; Revised 24 December 2013; Accepted 24 December 2013; Published 16 January 2014 AcademicEditor:MarcusJ.Schultz Copyright © 2014 Mattia Arrigo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Atrial fibrillation (AF) is common in ICU patients and is associated with a two- to fivefold increase in mortality. This paper provides a reappraisal of the management of AF with a special focus on critically ill patients with haemodynamic instability. AF can cause hypotension and heart failure with subsequent organ dysfunction. The underlying mechanisms are the loss of atrial contraction and the high ventricular rate. In unstable patients, sinus rhythm must be rapidly restored by synchronised electrical cardioversion (ECV). If pharmacological treatment is indicated, clinicians can choose between the rate control and the rhythm control strategy. The optimal substance should be selected depending on its potential adverse effects. A beta-1 antagonist with a very short half-life (e.g., esmolol) is an advantage for ICU patients because the effect of beta-blockade on cardiovascular stability is unpredictable in those patients. -

The Effect of Esmolol on Corrected-QT Interval, Corrected-QT
Rev Bras Anestesiol. 2015;65(1):34---40 REVISTA BRASILEIRA DE Official Publication of the Brazilian Society of Anesthesiology ANESTESIOLOGIA www.sba.com.br SCIENTIFIC ARTICLE The effect of esmolol on corrected-QT interval, corrected-QT interval dispersion changes seen during anesthesia induction in hypertensive patients taking an angiotensin-converting enzyme inhibitor ∗ Zahit C¸eker, Suna Akın Takmaz , Bülent Baltaci, Hülya Bas¸ar Department of Anesthesiolgy and Reanimation, Ankara Training and Research Hospital, Ministry of Health, Ankara, Turkey Received 16 January 2014; accepted 19 March 2014 Available online 1 May 2014 KEYWORDS Abstract Esmolol; Background and objectives: The importance of minimizing the exaggerated sympatho- adrenergic responses and QT interval and QT interval dispersion changes that may develop QT interval; due to laryngoscopy and tracheal intubation during anesthesia induction in the hypertensive QT dispersion; patients is clear. Esmolol decreases the hemodynamic response to laryngoscopy and intubation. ACE inhibitor However, the effect of esmolol in decreasing the prolonged QT interval and QT interval disper- sion as induced by laryngoscopy and intubation is controversial. We investigated the effect of esmolol on the hemodynamic, and corrected-QT interval and corrected-QT interval dispersion changes seen during anesthesia induction in hypertensive patients using angiotensin converting enzyme inhibitors. Methods: 60 ASA I---II patients, with essential hypertension using angiotensin converting enzyme inhibitors were included in the study. The esmolol group received esmolol at a bolus dose of 500 mcg/kg followed by a 100 mcg/kg/min infusion which continued until the 4th min after intubation. The control group received 0.9% saline similar to the esmolol group. -

Mechanism of Drug-Induced Qt Interval Prolongation
ABSTRACT Torsades de pointes (TdP) is a polymorphic ventricular tachycardia characterized by a distinctive pattern of undulating QRS complexes that twist around the isoelectric line. TdP is usually self-terminating or can subsequently degenerate into ventricular fibrillation, syncope, and sudden death. TdP has been associated with QT interval prolongation of the electrocardiogram; therefore, the QT interval has come to be recognized as a surrogate marker for the risk of TdP. International guidelines have been developed to harmonize both the preclinical and clinical studies for the evaluation of drug-induced TdP. However, currently preclinical in vitro and in vivo methods as well as biomarkers for proarrhythmias have been imperfect in predicting drug-induced TdP in humans. It is clear that relevant biomarkers together with appropriate models are needed to assess the arrhythmic risk of new chemical entities. The goal of the present dissertation is to create rabbit with myocardial failing heart as an in vivo animal model to predict TdP in humans and to determine mechanism(s) underlying TdP in this model. Electrocardiograms were recorded from bipolar transthoracic leads in 7 conscious healthy rabbits previously trained to rest quietly in slings. The RR and QT relationship, ii QT=2.4RR0.72 (r2=0.79, p < 0.001) was obtained by slowed the heart rate with 2.0 mg/kg zatebradine, and the algorithm for removing effect of heart rate on QT is QTc = QT/(RR)0.72. QTc lengthened significantly in all conscious rabbits given intravenous cisapride, dofetilide or haloperidol (p < 0.05), and QTc did not change with DMSO (vehicle control), propranolol or enalaprilat. -

1 the New Definition of Early Repolarisation Peter W. Macfarlane
1 The New Definition of Early Repolarisation Peter W. Macfarlane Institute of Cardiovascular and Medical Sciences University of Glasgow Address for Correspondence: Electrocardiology Group Level 1, New Lister Building Royal Infirmary, Glasgow G31 2ER Scotland, UK. e-mail: [email protected] 2 Introduction There has been an increasing interest in the electrocardiographic pattern of early repolarization in recent years following the publication of a paper by Haissaguerre and colleagues [1] in 2008, which linked their definition of the pattern to sudden cardiac arrest due to idiopathic ventricular fibrillation. However, earlier and later publications [2-17] by other groups on the topic of early repolarisation seemed to exhibit disagreement over what constituted the ECG pattern of early repolarisation suggesting a degree of confusion following the 2008 publication [1]. In 1947, Myers et al [2] reported that, among other features, an “elevation of the RS-T take off of 0.5 - 2.0mm may be considered as a normal variant” provided that the ST segment was concave upwards and followed by a tall T wave. In 1951, Goldman also reported on RS-T segment elevation in V3-V6 as a normal variant [3]. The original description of what for many years was regarded as the early repolarization pattern can probably be traced back to an early paper by Wasserburger and Alt [4] dealing with normal RS-T elevation in the relatively newly established full 12 lead ECG. The authors pointed out that the ST elevation mostly arose from a distinct notch on the downslope of the R wave but was occasionally associated with a “well-defined slur”. -

The Short QT Syndrome
The Short QT Syndrome A note from the SADS Foundation References We provide this information with the hope that informing 1. Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman A Guide for Patients BR, Bjerregaard P. Idiopathic short QT interval: a new clinical physicians and other health care providers, as well as the public, and Health Care will encourage early and correct diagnosis and proper therapy syndrome? Cardiology 2000;94:99 –102. for congenital short QT syndrome (SQTS), resulting in the 2. Giustetto C, Di Monte F. Wolpert C, Borggrefe M, Schimpf R, Providers reduction and ultimately elimination of sudden cardiac arrest Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M, Gaita F. (SCA) and sudden cardiac death (SCD). Short QTsyndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 2006;27:2440 –2447. 3. Gaita F, Giustetto C, Bianchi F, Wolptert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT syndrome: a familial What do Patients and Parents Need to Know cause of sudden death. Circulation 2003;108:965–970. about SQTS? 4. Dhutia H, Malhotra A, Parpia S, et al. The Prevalence and • The warning signs and symptoms of SQTS. significance of a short QT interval in 18 825 low-risk individuals • Who to see for proper testing. including athletes. Br J Sports Med 2015;0:1-6 • How to protect their children and themselves. • How to expand their family pedigree and contact other family members who may be at risk What do Physicians need to know? • When to consider SQTS as a possible diagnosis. -
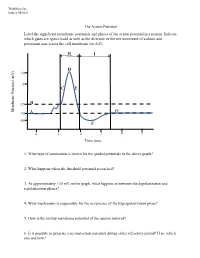
The Action Potential Label the Significant Membrane Potentials and Phases of the Action Potential in a Neuron
Worksheet by Andrey Michel The Action Potential Label the significant membrane potentials and phases of the action potential in a neuron. Indicate which gates are open/closed as well as the direction of the net movement of sodium and potassium ions across the cell membrane for A-G. H I D +30 0 C E -55 B Membrane Potential Membrane (mV) G -70 A -80 F 0 1 2 3 4 5 Time (ms) 1. What type of summation is shown for the graded potentials in the above graph? 2. What happens when the threshold potential is reached? 3. At approximately +30 mV on the graph, what happens in between the depolarization and repolarization phases? 4. What mechanism is responsible for the occurrence of the hyperpolarization phase? 5. How is the resting membrane potential of the neuron restored? 6. Is it possible to generate a second action potential during either refractory period? If so, which one and how? Place the following events in chronological order from 1-8: Na+ enters the cell, and depolarization occurs to approximately +30 mV. The voltage across the cell membrane is -70 mV, the resting membrane potential. Upon reaching the peak of the action potential, the VG Na+ channels are inactivated by the closing of their inactivation gate and the activation gate of each VG K+ channel opens. VG K+ channels close by the closing of their activation gate, and the resting membrane potential is gradually restored. An excitatory post-synaptic potential depolarizes the membrane to threshold and the activation gate of VG Na+ channels open. -

Hyperhomocysteinemia Alters Sinoatrial and Atrioventricular Nodal Function : Role of the Cardiac NMDA Receptor
University of Louisville ThinkIR: The University of Louisville's Institutional Repository Electronic Theses and Dissertations 12-2012 Hyperhomocysteinemia alters sinoatrial and atrioventricular nodal function : role of the cardiac NMDA receptor. Chirag V. Soni University of Louisville Follow this and additional works at: https://ir.library.louisville.edu/etd Recommended Citation Soni, Chirag V., "Hyperhomocysteinemia alters sinoatrial and atrioventricular nodal function : role of the cardiac NMDA receptor." (2012). Electronic Theses and Dissertations. Paper 1360. https://doi.org/10.18297/etd/1360 This Doctoral Dissertation is brought to you for free and open access by ThinkIR: The University of Louisville's Institutional Repository. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of ThinkIR: The University of Louisville's Institutional Repository. This title appears here courtesy of the author, who has retained all other copyrights. For more information, please contact [email protected]. HYPERHOMOCYSTEINEMIA ALTERS SINOATRIAL AND ATRIOVENTRICULAR NODAL FUNCTION: ROLE OF THE CARDIAC NMDA RECEPTOR By Chi rag V. Soni B.A., Northwestern University, 1999 M.S., University of Louisville, 2005 A Dissertation Submitted to the Faculty of the School of Medicine of the University of Louisville In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy Department of Physiology and Biophysics University of Louisville Louisville, KY December 2012 Copyright 2012 by Chirag V. Soni All rights reserved HYPERHOMOCYSTEINEMIA ALTERS SINOATRIAL AND ATRIOVENTRICULAR NODAL FUNCTION: ROLE OF THE CARDIAC NMDA RECEPTOR By Chirag V. Soni B.A., Northwestern University, 1999 M.S., University of Louisville, 2005 A Dissertation Approved on August 27th, 2012 By the Following Dissertation Committee: Claudio Maldonado, Ph.D., Mentor Suresh Tyagi, Ph.D.