Section 15-3: Constant Volume and Constant Pressure Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analytic Geometry
Guide Study Georgia End-Of-Course Tests Georgia ANALYTIC GEOMETRY TABLE OF CONTENTS INTRODUCTION ...........................................................................................................5 HOW TO USE THE STUDY GUIDE ................................................................................6 OVERVIEW OF THE EOCT .........................................................................................8 PREPARING FOR THE EOCT ......................................................................................9 Study Skills ........................................................................................................9 Time Management .....................................................................................10 Organization ...............................................................................................10 Active Participation ...................................................................................11 Test-Taking Strategies .....................................................................................11 Suggested Strategies to Prepare for the EOCT ..........................................12 Suggested Strategies the Day before the EOCT ........................................13 Suggested Strategies the Morning of the EOCT ........................................13 Top 10 Suggested Strategies during the EOCT .........................................14 TEST CONTENT ........................................................................................................15 -
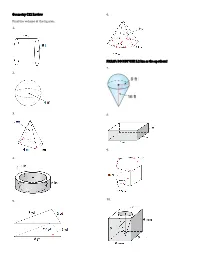
Geometry C12 Review Find the Volume of the Figures. 1. 2. 3. 4. 5. 6
Geometry C12 Review 6. Find the volume of the figures. 1. PREAP: DO NOT USE 5.2 km as the apothem! 7. 2. 3. 8. 9. 4. 10. 5. 11. 16. 17. 12. 18. 13. 14. 19. 15. 20. 21. A sphere has a volume of 7776π in3. What is the radius 33. Two prisms are similar. The ratio of their volumes is of the sphere? 8:27. The surface area of the smaller prism is 72 in2. Find the surface area of the larger prism. 22a. A sphere has a volume of 36π cm3. What is the radius and diameter of the sphere? 22b. A sphere has a volume of 45 ft3. What is the 34. The prisms are similar. approximate radius of the sphere? a. What is the scale factor? 23. The scale factor (ratio of sides) of two similar solids is b. What is the ratio of surface 3:7. What are the ratios of their surface areas and area? volumes? c. What is the ratio of volume? d. Find the volume of the 24. The scale factor of two similar solids is 12:5. What are smaller prism. the ratios of their surface areas and volumes? 35. The prisms are similar. 25. The scale factor of two similar solids is 6:11. What are a. What is the scale factor? the ratios of their surface areas and volumes? b. What is the ratio of surface area? 26. The ratio of the volumes of two similar solids is c. What is the ratio of 343:2197. What is the scale factor (ratio of sides)? volume? d. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -

HEAT and TEMPERATURE Heat Is a Type of ENERGY. When Absorbed
HEAT AND TEMPERATURE Heat is a type of ENERGY. When absorbed by a substance, heat causes inter-particle bonds to weaken and break which leads to a change of state (solid to liquid for example). Heat causing a phase change is NOT sufficient to cause an increase in temperature. Heat also causes an increase of kinetic energy (motion, friction) of the particles in a substance. This WILL cause an increase in TEMPERATURE. Temperature is NOT energy, only a measure of KINETIC ENERGY The reason why there is no change in temperature at a phase change is because the substance is using the heat only to change the way the particles interact (“stick together”). There is no increase in the particle motion and hence no rise in temperature. THERMAL ENERGY is one type of INTERNAL ENERGY possessed by an object. It is the KINETIC ENERGY component of the object’s internal energy. When thermal energy is transferred from a hot to a cold body, the term HEAT is used to describe the transferred energy. The hot body will decrease in temperature and hence in thermal energy. The cold body will increase in temperature and hence in thermal energy. Temperature Scales: The K scale is the absolute temperature scale. The lowest K temperature, 0 K, is absolute zero, the temperature at which an object possesses no thermal energy. The Celsius scale is based upon the melting point and boiling point of water at 1 atm pressure (0, 100o C) K = oC + 273.13 UNITS OF HEAT ENERGY The unit of heat energy we will use in this lesson is called the JOULE (J). -

Heat Energy a Science A–Z Physical Series Word Count: 1,324 Heat Energy
Heat Energy A Science A–Z Physical Series Word Count: 1,324 Heat Energy Written by Felicia Brown Visit www.sciencea-z.com www.sciencea-z.com KEY ELEMENTS USED IN THIS BOOK The Big Idea: One of the most important types of energy on Earth is heat energy. A great deal of heat energy comes from the Sun’s light Heat Energy hitting Earth. Other sources include geothermal energy, friction, and even living things. Heat energy is the driving force behind everything we do. This energy gives us the ability to run, dance, sing, and play. We also use heat energy to warm our homes, cook our food, power our vehicles, and create electricity. Key words: cold, conduction, conductor, convection, energy, evaporate, fire, friction, fuel, gas, geothermal heat, geyser, heat energy, hot, insulation, insulator, lightning, liquid, matter, particles, radiate, radiant energy, solid, Sun, temperature, thermometer, transfer, volcano Key comprehension skill: Cause and effect Other suitable comprehension skills: Compare and contrast; classify information; main idea and details; identify facts; elements of a genre; interpret graphs, charts, and diagram Key reading strategy: Connect to prior knowledge Other suitable reading strategies: Ask and answer questions; summarize; visualize; using a table of contents and headings; using a glossary and bold terms Photo Credits: Front cover: © iStockphoto.com/Julien Grondin; back cover, page 5: © iStockphoto.com/ Arpad Benedek; title page, page 20 (top): © iStockphoto.com/Anna Ziska; pages 3, 9, 20 (bottom): © Jupiterimages Corporation; -

IB Questionbank
Topic 3 Past Paper [94 marks] This question is about thermal energy transfer. A hot piece of iron is placed into a container of cold water. After a time the iron and water reach thermal equilibrium. The heat capacity of the container is negligible. specific heat capacity. [2 marks] 1a. Define Markscheme the energy required to change the temperature (of a substance) by 1K/°C/unit degree; of mass 1 kg / per unit mass; [5 marks] 1b. The following data are available. Mass of water = 0.35 kg Mass of iron = 0.58 kg Specific heat capacity of water = 4200 J kg–1K–1 Initial temperature of water = 20°C Final temperature of water = 44°C Initial temperature of iron = 180°C (i) Determine the specific heat capacity of iron. (ii) Explain why the value calculated in (b)(i) is likely to be different from the accepted value. Markscheme (i) use of mcΔT; 0.58×c×[180-44]=0.35×4200×[44-20]; c=447Jkg-1K-1≈450Jkg-1K-1; (ii) energy would be given off to surroundings/environment / energy would be absorbed by container / energy would be given off through vaporization of water; hence final temperature would be less; hence measured value of (specific) heat capacity (of iron) would be higher; This question is in two parts. Part 1 is about ideal gases and specific heat capacity. Part 2 is about simple harmonic motion and waves. Part 1 Ideal gases and specific heat capacity State assumptions of the kinetic model of an ideal gas. [2 marks] 2a. two Markscheme point molecules / negligible volume; no forces between molecules except during contact; motion/distribution is random; elastic collisions / no energy lost; obey Newton’s laws of motion; collision in zero time; gravity is ignored; [4 marks] 2b. -

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -

Chapter 1: Analytic Geometry
1 Analytic Geometry Much of the mathematics in this chapter will be review for you. However, the examples will be oriented toward applications and so will take some thought. In the (x,y) coordinate system we normally write the x-axis horizontally, with positive numbers to the right of the origin, and the y-axis vertically, with positive numbers above the origin. That is, unless stated otherwise, we take “rightward” to be the positive x- direction and “upward” to be the positive y-direction. In a purely mathematical situation, we normally choose the same scale for the x- and y-axes. For example, the line joining the origin to the point (a,a) makes an angle of 45◦ with the x-axis (and also with the y-axis). In applications, often letters other than x and y are used, and often different scales are chosen in the horizontal and vertical directions. For example, suppose you drop something from a window, and you want to study how its height above the ground changes from second to second. It is natural to let the letter t denote the time (the number of seconds since the object was released) and to let the letter h denote the height. For each t (say, at one-second intervals) you have a corresponding height h. This information can be tabulated, and then plotted on the (t, h) coordinate plane, as shown in figure 1.0.1. We use the word “quadrant” for each of the four regions into which the plane is divided by the axes: the first quadrant is where points have both coordinates positive, or the “northeast” portion of the plot, and the second, third, and fourth quadrants are counted off counterclockwise, so the second quadrant is the northwest, the third is the southwest, and the fourth is the southeast. -

TEMPERATURE, HEAT, and SPECIFIC HEAT INTRODUCTION Temperature and Heat Are Two Topics That Are Often Confused
TEMPERATURE, HEAT, AND SPECIFIC HEAT INTRODUCTION Temperature and heat are two topics that are often confused. Temperature measures how hot or cold an object is. Commonly this is measured with the aid of a thermometer even though other devices such as thermocouples and pyrometers are also used. Temperature is an intensive property; it does not depend on the amount of material present. In scientific work, temperature is most commonly expressed in units of degrees Celsius. On this scale the freezing point of water is 0oC and its boiling point is 100oC. Heat is a form of energy and is a phenomenon that has its origin in the motion of particles that make up a substance. Heat is an extensive property. The unit of heat in the metric system is called the calorie (cal). One calorie is defined as the amount of heat necessary to raise 1 gram of water by 1oC. This means that if you wish to raise 7 g of water by 4oC, (4)(7) = 28 cal would be required. A somewhat larger unit than the calorie is the kilocalorie (kcal) which equals 1000 cal. The definition of the calorie was made in reference to a particular substance, namely water. It takes 1 cal to raise the temperature of 1 g of water by 1oC. Does this imply perhaps that the amount of heat energy necessary to raise 1 g of other substances by 1oC is not equal to 1 cal? Experimentally we indeed find this to be true. In the modern system of international units heat is expressed in joules and kilojoules. -

Hydraulics Manual Glossary G - 3
Glossary G - 1 GLOSSARY OF HIGHWAY-RELATED DRAINAGE TERMS (Reprinted from the 1999 edition of the American Association of State Highway and Transportation Officials Model Drainage Manual) G.1 Introduction This Glossary is divided into three parts: · Introduction, · Glossary, and · References. It is not intended that all the terms in this Glossary be rigorously accurate or complete. Realistically, this is impossible. Depending on the circumstance, a particular term may have several meanings; this can never change. The primary purpose of this Glossary is to define the terms found in the Highway Drainage Guidelines and Model Drainage Manual in a manner that makes them easier to interpret and understand. A lesser purpose is to provide a compendium of terms that will be useful for both the novice as well as the more experienced hydraulics engineer. This Glossary may also help those who are unfamiliar with highway drainage design to become more understanding and appreciative of this complex science as well as facilitate communication between the highway hydraulics engineer and others. Where readily available, the source of a definition has been referenced. For clarity or format purposes, cited definitions may have some additional verbiage contained in double brackets [ ]. Conversely, three “dots” (...) are used to indicate where some parts of a cited definition were eliminated. Also, as might be expected, different sources were found to use different hyphenation and terminology practices for the same words. Insignificant changes in this regard were made to some cited references and elsewhere to gain uniformity for the terms contained in this Glossary: as an example, “groundwater” vice “ground-water” or “ground water,” and “cross section area” vice “cross-sectional area.” Cited definitions were taken primarily from two sources: W.B.