Nitrinergic Neurons in the Developing and Adult Human Telencephalon: Transient and Permanent Patterns of Expression in Comparison to Other Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Abnormalities of Grey and White Matter [11C]Flumazenil Binding In](https://docslib.b-cdn.net/cover/7913/abnormalities-of-grey-and-white-matter-11c-flumazenil-binding-in-217913.webp)
Abnormalities of Grey and White Matter [11C]Flumazenil Binding In
Brain (2002), 125, 2257±2271 Abnormalities of grey and white matter [11C]¯umazenil binding in temporal lobe epilepsy with normal MRI A. Hammers,1,2,3 M. J. Koepp,1,2,3 R. Hurlemann,2 M. Thom,2 M. P. Richardson,1,2,3 D. J. Brooks1 and J. S. Duncan1,2,3 1MRC Clinical Sciences Centre and Division of Correspondence to: Professor John S. Duncan, MA, DM, Neuroscience, Faculty of Medicine, Imperial College, FRCP, National Society for Epilepsy and Institute of 2Department of Clinical and Experimental Epilepsy, Neurology, 33 Queen Square, London WC1N 3BG, UK Institute of Neurology, University College London, London E-mail: [email protected] and 3National Society for Epilepsy MRI Unit, Chalfont St Peter, UK Summary In 20% of potential surgical candidates with refrac- the 16 patients with abnormalities, ®ndings were con- tory epilepsy, current optimal MRI does not identify cordant with EEG and clinical data, enabling further the cause. GABA is the principal inhibitory neuro- presurgical evaluation. Group ®ndings were: (i) transmitter in the brain, and GABAA receptors are decreased FMZ-Vd in the ipsilateral (Z = 3.01) and expressed by most neurones. [11C]Flumazenil (FMZ) contralateral (Z = 2.56) hippocampus; (ii) increased PET images the majority of GABAA receptor sub- FMZ-Vd in the ipsilateral (Z = 3.71) and contralat- types. We investigated abnormalities of FMZ binding eral TLWM (two clusters, Z = 3.11 and 2.79); and in grey and white matter in 18 patients with refrac- (iii) increased FMZ-Vd in the ipsilateral frontal lobe tory temporal lobe epilepsy (TLE) and normal quan- white matter between the superior and medial frontal titative MRI. -

Corticostriatal Interactions During Learning, Memory Processing, and Decision Making
The Journal of Neuroscience, October 14, 2009 • 29(41):12831–12838 • 12831 Mini-Symposium Corticostriatal Interactions during Learning, Memory Processing, and Decision Making Cyriel M. A. Pennartz,1 Joshua D. Berke,2,3 Ann M. Graybiel,4,5 Rutsuko Ito,6 Carien S. Lansink,1 Matthijs van der Meer,7 A. David Redish,7 Kyle S. Smith,4,5 and Pieter Voorn8 1University of Amsterdam, Swammerdam Institute for Life Sciences Center for Neuroscience, 1098 XH Amsterdam, The Netherlands, 2Department of Psychology and 3Neuroscience Program, University of Michigan, Ann Arbor, Michigan 48109, 4Department of Brain and Cognitive Sciences and 5McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, 6Department of Experimental Psychology, University of Oxford, Oxford OX1 3UD, United Kingdom, 7Department of Neuroscience, University of Minnesota, Minneapolis, Minnesota 55455, and 8Department of Anatomy, Research Institute Neurosciences, Vrije Universiteit University Medical Center, 1007 MB Amsterdam, The Netherlands This mini-symposium aims to integrate recent insights from anatomy, behavior, and neurophysiology, highlighting the anatom- ical organization, behavioral significance, and information-processing mechanisms of corticostriatal interactions. In this sum- mary of topics, which is not meant to provide a comprehensive survey, we will first review the anatomy of corticostriatal circuits, comparing different ways by which “loops” of cortical–basal ganglia circuits communicate. Next, we will address the causal importance and systems-neurophysiological mechanisms of corticostriatal interactions for memory, emphasizing the communi- cation between hippocampus and ventral striatum during contextual conditioning. Furthermore, ensemble recording techniques have been applied to compare information processing in the dorsal and ventral striatum to predictions from reinforcement learning theory. -

Cortical Layers: What Are They Good For? Neocortex
Cortical Layers: What are they good for? Neocortex L1 L2 L3 network input L4 computations L5 L6 Brodmann Map of Cortical Areas lateral medial 44 areas, numbered in order of samples taken from monkey brain Brodmann, 1908. Primary visual cortex lamination across species Balaram & Kaas 2014 Front Neuroanat Cortical lamination: not always a six-layered structure (archicortex) e.g. Piriform, entorhinal Larriva-Sahd 2010 Front Neuroanat Other layered structures in the brain Cerebellum Retina Complexity of connectivity in a cortical column • Paired-recordings and anatomical reconstructions generate statistics of cortical connectivity Lefort et al. 2009 Information flow in neocortical microcircuits Simplified version “computational Layer 2/3 layer” Layer 4 main output Layer 5 main input Layer 6 Thalamus e - excitatory, i - inhibitory Grillner et al TINS 2005 The canonical cortical circuit MAYBE …. DaCosta & Martin, 2010 Excitatory cell types across layers (rat S1 cortex) Canonical models do not capture the diversity of excitatory cell classes Oberlaender et al., Cereb Cortex. Oct 2012; 22(10): 2375–2391. Coding strategies of different cortical layers Sakata & Harris, Neuron 2010 Canonical models do not capture the diversity of firing rates and selectivities Why is the cortex layered? Do different layers have distinct functions? Is this the right question? Alternative view: • When thinking about layers, we should really be thinking about cell classes • A cells class may be defined by its input connectome and output projectome (and some other properties) • The job of different classes is to (i) make associations between different types of information available in each cortical column and/or (ii) route/gate different streams of information • Layers are convenient way of organising inputs and outputs of distinct cell classes Excitatory cell types across layers (rat S1 cortex) INTRATELENCEPHALIC (IT) | PYRAMIDAL TRACT (PT) | CORTICOTHALAMIC (CT) From Cereb Cortex. -

Perspectives
PERSPECTIVES reptiles, to birds and mammals, to primates OPINION and, finally, to humans — ascending from ‘lower’ to ‘higher’ intelligence in a chrono- logical series. They believed that the brains Avian brains and a new understanding of extant vertebrates retained ancestral structures, and, therefore, that the origin of of vertebrate brain evolution specific human brain subdivisions could be traced back in time by examining the brains of extant non-human vertebrates. In The Avian Brain Nomenclature Consortium* making such comparisons, they noted that the main divisions of the human CNS — Abstract | We believe that names have a pallium is nuclear, and the mammalian the spinal cord, hindbrain, midbrain, thala- powerful influence on the experiments we cortex is laminar in organization, the avian mus, cerebellum and cerebrum or telen- do and the way in which we think. For this pallium supports cognitive abilities similar cephalon — were present in all vertebrates reason, and in the light of new evidence to, and for some species more advanced than, (FIG. 1a). Edinger, however, noted that the about the function and evolution of the those of many mammals. To eliminate these internal organization of the telencephala vertebrate brain, an international consortium misconceptions, an international forum of showed the most pronounced differences of neuroscientists has reconsidered the neuroscientists (BOX 1) has, for the first time between species. In mammals, the outer traditional, 100-year-old terminology that is in 100 years, developed new terminology that part of the telencephalon was found to have used to describe the avian cerebrum. Our more accurately reflects our current under- prominently layered grey matter (FIG. -

The Ventral Striatum in Off-Line Processing: Ensemble Reactivation During Sleep and Modulation by Hippocampal Ripples
6446 • The Journal of Neuroscience, July 21, 2004 • 24(29):6446–6456 Behavioral/Systems/Cognitive The Ventral Striatum in Off-Line Processing: Ensemble Reactivation during Sleep and Modulation by Hippocampal Ripples C. M. A. Pennartz,1 E. Lee,1 J. Verheul,1 P. Lipa,2 C. A. Barnes,2 and B. L. McNaughton2 1Graduate School of Neurosciences Amsterdam, University of Amsterdam, Faculty of Science, Swammerdam Institute for Life Sciences, 1090 GB, Amsterdam, The Netherlands, and 2Arizona Research Laboratories Division of Neural Systems, Memory, and Aging, University of Arizona, Tucson, Arizona 85724 Previously it has been shown that the hippocampus and neocortex can spontaneously reactivate ensemble activity patterns during post-behavioral sleep and rest periods. Here we examined whether such reactivation also occurs in a subcortical structure, the ventral striatum, which receives a direct input from the hippocampal formation and has been implicated in guidance of consummatory and conditioned behaviors. During a reward-searching task on a T-maze, flanked by sleep and rest periods, parallel recordings were made from ventral striatal ensembles while EEG signals were derived from the hippocampus. Statistical measures indicated a significant amount of reactivation in the ventral striatum. In line with hippocampal data, reactivation was especially prominent during post- behavioralslow-wavesleep,butunlikethehippocampus,nodecayinpatternrecurrencewasvisibleintheventralstriatumacrossthefirst 40 min of post-behavioral rest. We next studied the relationship between ensemble firing patterns in ventral striatum and hippocampal ripples–sharp waves, which have been implicated in pattern replay. Firing rates were significantly modulated in close temporal associ- ation with hippocampal ripples in 25% of the units, showing a marked transient enhancement in the average response profile. -

REVIEW Hormones and the Hippocampus
205 REVIEW Hormones and the hippocampus R Lathe Centre for Genome Research and Centre for Neuroscience, University of Edinburgh, West Mains Road, Edinburgh EH9 3JQ, UK (Requests for offprints should be addressed to R Lathe, Centre for Genome Research, King’s Buildings, West Mains Road, Edinburgh EH9 3JQ, UK; Email: [email protected]) Abstract Hippocampal lesions produce memory deficits, but the Functionally, hippocampal enteroception may reflect feed- exact function of the hippocampus remains obscure. back control; evidence is reviewed that the hippocampus Evidence is presented that its role in memory may be modulates body physiology, including the activity of the ancillary to physiological regulation. Molecular studies hypothalamus–pituitary–adrenal axis, blood pressure, demonstrate that the hippocampus is a primary target for immunity, and reproductive function. It is suggested that ligands that reflect body physiology, including ion balance the hippocampus operates, in parallel with the amygdala, and blood pressure, immunity, pain, reproductive status, to modulate body physiology in response to cognitive satiety and stress. Hippocampal receptors are functional, stimuli. Hippocampal outputs are predominantly inhibi- probably accessible to their ligands, and mediate physio- tory on downstream neuroendocrine activity; increased logical and cognitive changes. This argues that an early synaptic efficacy in the hippocampus (e.g. long-term role of the hippocampus may have been in sensing soluble potentiation) could facilitate throughput inhibition. -

Olfactory Cortex Development
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Review | Development Development and organization of the evolutionary conserved three-layered olfactory cortex Olfactory cortex development Esther Klingler Department of Basic Neuroscience, University of Geneva, Geneva, Switzerland. DOI: 10.1523/ENEURO.0193-16.2016 Received: 6 July 2016 Revised: 11 November 2016 Accepted: 8 December 2016 Published: 20 January 2017 Author Contributions: EK designed and wrote the paper. Conflict of Interest: Authors report no conflict of interest. Correspondence should be addressed to Esther Klingler, Jabaudon Lab, Department of Basic Neuroscience, University of Geneva, CMU, 1, rue Michel Servet, 1206 Geneva – Switzerland, E-mail: [email protected] Cite as: eNeuro 2017; 10.1523/ENEURO.0193-16.2016 Alerts: Sign up at eneuro.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Accepted manuscripts are peer-reviewed but have not been through the copyediting, formatting, or proofreading process. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. Copyright © 2017 the authors ϭ Manuscript Title Page Ϯ ϯ ϰ 1. Manuscript Title ϱ Ǧ Ǥ ϲ ϳ 2. Abbreviated Title ϴ ϵ ϭϬ 3. List of all Author Names and Affiliations ϭϭ ǣ ǡ ǡ ǡǤ ϭϮ ϭϯ 4. Author Contributions ϭϰ Ǥ ϭϱ ϭϲ 5. Correspondence should be addressed to ϭϳ ǡǤ̷Ǥ ϭϴ ΪͶͳʹʹ͵ͻͶͳ ϭϵ ϮϬ ǡ ǡ Ϯϭ ͳǡ ϮϮ ͳʹͲ Ǧ Ϯϯ Ϯϰ 6. -

Satb2 Is Required for the Regionalization of Retrosplenial Cortex
Cell Death & Differentiation (2020) 27:1604–1617 https://doi.org/10.1038/s41418-019-0443-1 ARTICLE Satb2 is required for the regionalization of retrosplenial cortex 1 2 1,2 1 1 3,4 1 Lei Zhang ● Ning-Ning Song ● Qiong Zhang ● Wan-Ying Mei ● Chun-Hui He ● Pengcheng Ma ● Ying Huang ● 1 3,4 1,5 1,2,6 Jia-Yin Chen ● Bingyu Mao ● Bing Lang ● Yu-Qiang Ding Received: 23 March 2019 / Revised: 10 October 2019 / Accepted: 11 October 2019 / Published online: 30 October 2019 © The Author(s) 2019. This article is published with open access Abstract The retrosplenial cortex (Rsp) is a transitional cortex located between the neocortex and archicortex, but the molecular mechanism specifying Rsp from the archicortex remains elusive. We here report that the transcription factor Satb2 is required for specifying Rsp identity during its morphogenesis. In Satb2 CKO mice, the boundary between the Rsp and archicortex [i.e., subiculum (SubC)] disappears as early as E17.5, and Rsp efferent projection is aberrant. Rsp-specific genes are lost, whereas SubC-specific genes are ectopically expressed in Rsp of Satb2 CKO mice. Furthermore, cell-autonomous role of Satb2 in maintaining Rsp neuron identity is revealed by inactivation of Satb2 in Rsp neurons. Finally, Satb2 represses the transcription of Nr4a2. The misexpression of Nr4a2 together with Ctip2 induces expression of SubC-specific genes in wild-type Rsp, and simultaneous knockdown of these two genes in Rsp Satb2-mutant cells prevents their fate transition to SubC identity. Thus, 1234567890();,: 1234567890();,: Satb2 serves as a determinant gene in the Rsp regionalization by repressing Nr4a2 and Ctip2 during cortical development. -

Arterial Patterns of the Rat Rhinencephalon and Related Structures
EXPEKIRIEN'TAI. NE~'ROI.OGY 49, 671-690 (1975) Arterial Patterns of the Rat Rhinencephalon and Related Structures PETER CoYLE1 Rccciz*cd J~r~w 7. 19i5 Course and distribution information on arteries in the rat rhinencephalon was not found in the literature. Such data are useful for designing experi- ments and interpreting findings, tracing nerve fibers on or to intracerebral vessels, and in considering routes for diffusion or transport of intracerebral injected agents. Adult rats were perfused with silicone rubber and many brains were cleared in glycerin. The major arteries to the olfactory bulb stem from the anterior cerebral artery. A middle cerebral arterial ramus could provide a collateral source. The septum receives supply exclusively from the anterior cerebral artery. A rostra1 lesion in the medial septum would most likely involve arteries supplying more caudal structures includ- ing hippocampal afferent and efferent fibers. No anastomoses between septal arteries or with middle or posterior cerebral arterial rami were observed. The cingulate cortex receives anterior cerebral arterial branches with the middle cerebral artery being a collateral source. The amygdala and over- lying cortex receive branches of the internal carotid and middle cerebral arteries. Transverse arteries in the hippocampal fissure stem from the longitudinal hippocampal artery, a branch of the posterior cerebral artery, to nourish the hippocampus and portions of the fascia dentata. Other branches supply the remainder of the fascia dentata, entorhinal and sub- icular structures, and certain vessels anastomose with middle cerebral arterial rami. A transverse artery occlusion would probably result in a lesion : No intracerebral arterial anastomoses were observed. -

Neuroanatomy of the Common Dolphin (Delphinus Delphis)As Revealed by Magnetic Resonance Imaging (MRI)
THE ANATOMICAL RECORD 268:411–429 (2002) Neuroanatomy of the Common Dolphin (Delphinus delphis)as Revealed by Magnetic Resonance Imaging (MRI) LORI MARINO,1* KEITH D. SUDHEIMER,2 D. ANN PABST,3 3 1 2,4 WILLIAM A. MCLELLAN, DAVID FILSOOF, AND JOHN I. JOHNSON 1Neuroscience and Behavioral Biology Program, Emory University, Atlanta, Georgia 2Radiology Department, Michigan State University, East Lansing, Michigan 3Department of Biological Sciences and Center for Marine Science, University of North Carolina at Wilmington, Wilmington, North Carolina 4Neuroscience Program, Michigan State University, East Lansing, Michigan ABSTRACT In this study, magnetic resonance (MR) images of the brain of an adult common dolphin (Delphinus delphis) were acquired in the coronal plane at 66 antero-posterior levels. From these scans a computer-generated set of resectioned virtual images in orthogonal planes was constructed using the programs VoxelView and VoxelMath (Vital Images, Inc., Michigan State Univ.). Sections in all three planes reveal major neuroanatomical struc- tures. These structures in the adult common dolphin brain are compared with those from a fetal common dolphin brain from a previously published study as well as with MR images of adult brains of other odontocetes. This study, like previous ones, demonstrates the utility of MR imaging (MRI) for comparative neuroanatomical investigations of dolphin brains. Anat Rec 268:411–429, 2002. © 2002 Wiley-Liss, Inc. Key words: common dolphin; neuroanatomy; magnetic reso- nance imaging; MRI; brain Compared with other mammalian brains, the cetacean Although extensive studies have been conducted on the brain is, in many respects, highly unusual. Morgane et al. brains of other odontocetes, such as the bottlenose dolphin (1980, p. -

Journal of Anatomy
JOURNAL OF ANATOMY A CONTRIBUTION TO THE MORPHOLOGY OF THE CORPUS STRIATUM BY RAYMOND A. DART, Demonstrator of Anatomy, University College, London INTRODUCTION Fou a recapitulation of the essential features in the divergent conclusions of investigators who have studied this problem, we are indebted to the recent paper by Elliot Smith ('20) in which he pointed out the nature of the corpus striatum in Sphenodon, and indicated the morphological relationships of its several parts. For some time past I have been studying a series of sections of the brain of the highly specialised Marsupial Mole, Notoryctes typhlops, which was very kindly placed at my disposal by Prof. Elliot Smith. As might be anticipated, in this creature devoid of any visual apparatus, the olfactory and closely associated striatal areas play a dominant role in its cerebral constitution-features already described by Elliot Smith in his communication to the Royal Society of South Australia ('95). In attempting to elucidate the significance of these structures I have investigated more primitive forms in the biological series; and I have to acknowledge the generosity of Professors A. Dendy of King's College and J. P. Hill of University College for the free access to their important collections, which has made possible an extensive comparative study. It is primarily for the purpose of abbreviating the account of the brain of Notoryctes that this preliminary note upon the striatal region is submitted; but the problem of the evolution of the corpus striatum is sufficiently important to call for this separate treatment. - If a transverse section of the brain of Notoryctes be studied in the region of the anterior commissure and foramen of Monro (e.g. -
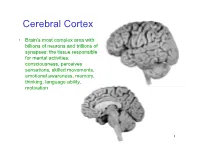
Cerebral Cortex
Cerebral Cortex • Brain’s most complex area with billions of neurons and trillions of synapses: the tissue responsible for mental activities: consciousness, perceives sensations, skilled movements, emotional awareness, memory, thinking, language ability, motivation 1 John Lorber, Science 210:1232 (1980) “There a young student at this university who has an IQ of 126, has gained a first-class honors degree in mathematics, and is socially completely normal. And yet the boy has virtually no brain.” “The student’s physician at the university noticed that the student had slightly larger than normal head… When we did a brain scan on him we saw that instead of the normal 4.5 cm thickness of brain tissue there was just a millimeter or so. His cranium is filled with CSF.” How is this possible? What does it tell us? Do you think this would be OK if it happened to an adult? To a 15 year old? To a 5 year old? To a neonate? 3 Types of Cerebral Cortex • Neocortex – Newest in evolution – About 90% of total – 6 layers, most complex • Paleocortex – Associated with olfactory system, the parahippocampal gyrus, uncus – fewer than 6 layers • Archicortex – Hippocampal formation; limbic system – 3 layers, most primitive • Mesocortex – Cingulate gyrus, insular cortex – Transitional between archicortex and neocortex 5 The perks of having a neocortex • The words used to describe the higher mental capacities of animals with a large neocortex include: – CONSCIOUSNESS – FREE WILL – INTELLIGENCE – INSIGHT • Animals with much simpler brains learn well, so LEARNING should not be among these capacities (Macphail 1982). • A species could have genetically determined mechanisms, acquired through evolutionary selection, for taking advantage of the regular features of the environment, or they could have learned through direct experience.