Abiotic Conditions, Algal Biomass & Fish Growth Rates Affect Fish Mercury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Small Community Youth Employment Support Brochure
We are Contact Us Youth Employment - Here to Help Regional ECE Service Centres: Small Community How do you apply? Fort Simpson (867) 695-7338 Dehcho Regional Education Centre Employment Support Contact your regional ECE Service Centre Developing workplace skills for more information. Fort Smith (867) 872-7425 Sweetgrass Building The Small Community Employment Support program provides wage subsidies to organizations in small NWT Hay River (867) 874-5050 communities who offer training in the workplace Courthouse Building to unemployed youth. Inuvik (867) 777-7365 GNWT Multi-use Building Norman Wells (867) 587-7157 Edward G. Hodgson Building Yellowknife (867) 766-5100 Nova Plaza www.ece.gov.nt.ca If you would like this information in another official language, call us. Am I Eligible? Eligible Communities Financial Assistance Employers must be: Only organizations in the following communities Wage subsidies are available for a 4 – 40 week • Licensed to operate in the Northwest Territories are eligible to apply for funding: employment period. • Located in an eligible community • Aklavik • Kakisa Wage subsidies are available only for fulltime positions (minimum of 30 hours per week). • In operation for at least 6 months • Colville Lake • Łutselk’e • Délįne • Nahanni Butte Wage subsidies apply only to regular hours worked by the • In good standing with the Workers’ Safety and trainee. All overtime, vacation, statutory holidays, sick and Compensation Commission • Dettah • Paulatuk special leave is the responsibility of the employer. • One of the following: • Enterprise • Sachs Harbour Employers must contribute a minimum of 20% of the trainee’s • Business or Corporation • Fort Good Hope • Trout Lake wage and pay at least minimum wage, including other benefits • Aboriginal Government or Organization • Fort Liard • Tsiigehtchic and dues found in the Employment Standards Act. -

Mercury Concentrations Appear to Be Increasing in Predatory Fish in Lakes
Spatial and temporal variability in mercury concentrations in predatory fish in lakes along the Mackenzie River and in Great Slave Lake Marlene Evans1, George Low2, Derek Muir3, Jonathan Keating1, Xiaowa Wang3, Mike Low2, Diane Giroux4, Mike Tollis5, and Shawn Buckley6 1Environment Canada, Saskatoon, SK; 2Dehcho First Nations, Hay River, NT; 3Environment Canada, Burlington, ON; 4Akaitcho Territory Government, Fort Resolution, NT; 5Lutsel K'e Dene First Nation, Lutsel K'e, NT; 6Hay River, NT 1.25 1.25 Willow Lake Big Island Lake McGill and Deep lakes 1999 2012 2 Abstract 1.00 2012 1.00 McGill Lake (3.6 km ): average Hg concentrations in 2000 were low in lake whitefish and white suckers, but high in walleye and northern pike. g/g) Our previous research on mercury concentrations in predatory fish in lakes 0.75 0.75 In 2010, Hg concentrations remained high in walleye and increased in Guideline for commercial along the Mackenzie River determined that concentrations tended to be sale of fish northern pike. highest in small lakes, particularly where fish were old (mean age > 10 0.50 0.50 Deep Lake (2.1 km2): average Hg concentrations in 2000 were Mercury ( years), and lowest in large lakes like Great Slave Lake (Evans et al. 2005; 0.25 0.25 moderately low in lake whitefish and walleye, but high in northern pike. Lockhart et al. 2005). Mercury continues to be of concern in northern In 2011, Hg concentrations were higher in walleye and northern pike. environments because of warming trends and increased mercury 0.00 0.00 lake lake northern lake lake northern burbot emissions from Asian sources, which may be reaching the NWT. -

Taiga Plains
ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES Taiga Plains Ecosystem Classification Group Department of Environment and Natural Resources Government of the Northwest Territories Revised 2009 ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES TAIGA PLAINS This report may be cited as: Ecosystem Classification Group. 2007 (rev. 2009). Ecological Regions of the Northwest Territories – Taiga Plains. Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, NT, Canada. viii + 173 pp. + folded insert map. ISBN 0-7708-0161-7 Web Site: http://www.enr.gov.nt.ca/index.html For more information contact: Department of Environment and Natural Resources P.O. Box 1320 Yellowknife, NT X1A 2L9 Phone: (867) 920-8064 Fax: (867) 873-0293 About the cover: The small photographs in the inset boxes are enlarged with captions on pages 22 (Taiga Plains High Subarctic (HS) Ecoregion), 52 (Taiga Plains Low Subarctic (LS) Ecoregion), 82 (Taiga Plains High Boreal (HB) Ecoregion), and 96 (Taiga Plains Mid-Boreal (MB) Ecoregion). Aerial photographs: Dave Downing (Timberline Natural Resource Group). Ground photographs and photograph of cloudberry: Bob Decker (Government of the Northwest Territories). Other plant photographs: Christian Bucher. Members of the Ecosystem Classification Group Dave Downing Ecologist, Timberline Natural Resource Group, Edmonton, Alberta. Bob Decker Forest Ecologist, Forest Management Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Hay River, Northwest Territories. Bas Oosenbrug Habitat Conservation Biologist, Wildlife Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, Northwest Territories. Charles Tarnocai Research Scientist, Agriculture and Agri-Food Canada, Ottawa, Ontario. Tom Chowns Environmental Consultant, Powassan, Ontario. Chris Hampel Geographic Information System Specialist/Resource Analyst, Timberline Natural Resource Group, Edmonton, Alberta. -

Hay River Region
The Hay Water RiverMonitoring Activities in the Hay River Region Kátåo’dehé at Enterprise Kátåo’dehé is the South Slavey Dene name for the Hay River. In Chipewyan, the Hay River is Hátå’oresche. In Cree, it is Maskosï-Sïpiy. The Hay River is a culturally significant river for Northerners and an integral part of the Mackenzie River Basin. Given its importance, there are several monitoring initiatives in the region designed to better understand the river and to detect changes. The information collected through these programs can also help to address questions that people may have. While amounts vary from year to year, the volume of water in the Hay River has remained relatively stable since monitoring began in 1963. Only a slight increasing trend in winter flow was revealed. Some changes in water quality were also found, such as increasing trends in phosphorus and decreasing trends in calcium, magnesium and sulphate. This means that the levels of these substances have changed since sampling began in 1988. Further work is needed to understand the ecological significance of these trends. Overall, the water quality and quantity of the Hay River is good. Continued monitoring activities will increase our knowledge of this important river and identify change. It is important that all water partners work closely together on any monitoring initiatives. 1 Water Monitoring Activities in the Hay River Region For information regarding reproduction rights, please contact Public Works and Government Services Canada at: (613) 996-6886 or at: [email protected] www.aandc.gc.ca 1-800-567-9604 TTY only 1-866-553-0554 QS-Y389-000-EE-A1 Catalogue: R3-206/2014E ISBN: 978-1-100-233343-7 © Her Majesty the Queen in right of Canada, represented by the Minister of Aboriginal Affairs and Northern Development Canada, 2014 This Publication is also available in French under the title: La Rivière Hay : activités de surveillance de l’eau dans la région de la rivière Hay. -
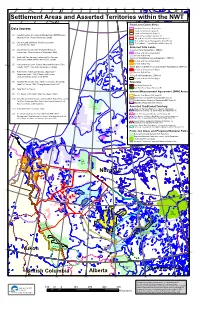
Settlement Areas and Asserted Territories Within The
160°W 155°W 150°W 145°W 140°W 135°W 130°W 125°W 120°W 115°W 110°W 105°W 100°W 95°W 90°W 85°W 80°W 75°W Settlement Areas and Asserted Territories within the NWT Final Land Claim Areas Inuvailuit Settlement Region (3) Data Sources: Gwich'in Settlement Area (2) Gwich'in Primary Use Area (2) (1) Canadian Centre for Cadastral Management, NWT/Nunavut Gwich'in Secondary Use Area (2) Regional Office, Natural Resources Canada Sahtu Dene and Métis Settlement Area (1) Tåîchô Wek 'èezhii Management Area (5) (12) (2) Gwich'in Land and Water Board, Data current Tåîchô Môwhi Gogha Dè Nîîtåèè Boundary (5) as of December, 2002 Selected Title Lands (3) Joint Secretariat Inuvialuit Renewable Resource Inuvialuit Final Agreement, 1984(1) Committees. Data current as of December, 2002. Surface and Sub-Surface Rights Surface Rights Only (4) Lands and Trust Services, Indian and Inuit Services Gwich'in Comprehensive Agreement, 1992 (1) Directorate, Indian and Northern Affairs Canada Surface and Sub-Surface Rights Surface Rights Only 75°N (5) Comprehensive Claims Branch, Indian and Northern Affairs Canada. NOTE: Data might be subject to change. Sahtu Dene and Métis Comprehensive Agreement, 1994 (1) Surface and Sub-Surface Rights 75°N (6) Prince Albert Tribal Council Study, Chipewyan – Surface Rights Only Denesuline Bands, 1990, ("Recent and Current Tåichô Final Agreement, 2005 (5) Land Use and Occupancy in the NWT") Surface and Sub-Surface Rights (7) Based on MKO Study, Sayisi Dene First Nation / Northlands Reserves Dene First Nation, 1993, ("Living Memory Land -

Communities and Diamonds Annual Report 2014
2014 Annual Report of the Government of the Northwest Territories under the Ekati, Diavik and Snap Lake Socio-Economic Agreements COMMUNITIES AND DIAMONDS PREPARED BY: Industry, Tourism and Investment, Socio-Economic Monitoring in the Education, Culture and Employment, Communities of Behchok, Detah, Finance, Health and Social Services, Gamètì, Łutselk’e, Ndilǫ, Wekweètì, Justice, Whatì and Yellowknife NWT Bureau of Statistics, NWT Housing Corporation March 2015 02 Communities and Diamonds 2014 To request a translation,To request please a translation, call the Department please call of theIndustry, Tourism and Department of Industry,Investment Tourism and Investment atat (867) (867) 920-8686. 920-8696 02 Communities and Diamonds 2014 Communities and Diamonds 2014 i Executive Summary The Communities and Diamonds Annual Report is produced partly in fulfillment of commitments made by the Government of the Northwest Territories (GNWT) in Socio-Economic Agreements (SEAs) with the mining companies operating in the Northwest Territories (NWT). There are currently three operating mines: the Dominion Diamond Corporation Ekati Mine, the Diavik Diamond Mine, and the De Beers Snap Lake Mine. The purpose of the Communities and Diamonds Report is to determine if, and how, mine activity may be affecting residents of Yellowknife and seven Small Local Communities (SLCs) in the NWT by examining socio-economic indicators for changes since 1996, when the first mine became operational. The seven SLCs are Behchok, Detah, Gamètì, Łutselk’e, Ndilǫ, Wekweètì, and Whatì. The Report examines the data based on the GNWT Sustainable Development Policy five-point framework: 1. community, family and individual well-being; 2. cultural well-being and traditional economy; 3. -

Survey of Exotic Plants Along NWT Highways (Oldham & Delisle‐Oldham 2017)
Report on the 2016 Survey of Exotic Plants along Northwest Territories Highways By Michael J. Oldham1 and Mireille Delisle-Oldham2 March 2017 1Ontario Natural Heritage Information Centre, Science and Research Branch, Ministry of Natural Resources and Forestry, 300 Water Street, Peterborough, Ontario K9L 1C8 2347 Plati Avenue, Peterborough, Ontario K9J 8M5 Report on the 2016 survey of exotic plants along NWT highways (Oldham & Delisle‐Oldham 2017) Table of Contents 1.0 Introduction …………………………………………………………………………. 4 2.0 Methodology ………………………………………………………………………… 8 2.1 Highway Survey Methodology …………………………………………… 9 2.2 Walking Survey Methodology ………………………………………….... 12 2.3 Territorial Park, Railway and Town Survey Methodology ………….. 13 3.0 Results ……………………………………………………………………………….. 14 3.1 Highway Surveys ……………………………………………………...…… 14 3.2 Walking Surveys ………………………………………………………...… 17 3.3 Territorial Park Surveys …………………………………………………... 19 3.4 Railway Surveys ……………………………………………………………. 25 3.5 Town Surveys ……………………………………………………………..... 27 3.6 Inuvik to Tuktoyaktuk Highway (ITH) Survey …………………………. 34 4.0 Acknowledgements ………………………………...……………………………… 37 5.0 Literature Cited and Bibliography of Floristic Literature Southern NWT ... 38 6.0 Appendices …………………………………………………………………..……... 45 List of Tables and Figures Tables Table 1. Number of records for exotic plants documented during 2006 and 2016 NWT surveys …………………………………………………………………………………. 5 Table 2. Priority invasive plant species for the 2016 NWT Exotic Plant Highways Survey …………………………………………………………………………………………. 9 Table 3. Territorial highways covered during 2016 exotic plant surveys …...…… 10 Table 4. Abundance categories for the 2016 NWT Exotic Plant Survey …………. 11 Table 5. 2016 highways surveys on each NWT territorial highway ………………. 14 2 Report on the 2016 survey of exotic plants along NWT highways (Oldham & Delisle‐Oldham 2017) Table 6. Exotic plant species detected on 2016 NWT highway surveys ………… 15 Table 7. Exotic plant species detected on 2016 NWT walking and highway surveys ………………………………………………………………………………………. -
Arctic Environmental Strategy Summary of Recent Aquatic Ecosystem Studies Northern Water Resources Studies
Arctic Environmental Strategy Summary of Recent Aquatic Ecosystem Studies Northern Water Resources Studies Arctic Environmental Strategy Summary ofRecent Aquatic Ecosystem Studies August 1995 Northern Affairs Program Edited by J. Chouinard D. Milburn Published under the authority of the Honourable Ronald A. Irwin, P.C., M.P., Minister of Indian Affairs and Northern Development Ottawa, 1995 QS-8507-030-EF-Al Catalogue No. R72-244/1-1995E ISBN 0-662-23939-3 © Minister of Public Works and Government Services Canada FOREWORD The Arctic Environmental Strategy (AES), announced in April 1991, is a six-year $100 million Green Plan initiative. The overall goal ofthe AES is to preserve and enhance the integrity, health, biodiversity and productivity ofour Arctic ecosystems for the benefit ofpresent and future generations. Four specific programs address some ofthe key environmental challenges: they are waste cleanup, contaminants, water management, and environment and economy integration. The programs are managed by the Northern Affairs Program ofthe Department of Indian Affairs and Northern Development (DIAND); however, there is a strong emphasis on partnerships with northern stakeholders including Native organizations, other federal departments and the territorial governments. The AES Action on Water Program specifically strives to enhance the protection ofnorthern freshwaters through improved knowledge and decision-making. Water Resources managers in the Yukon and the Northwest Territories administer this Program which focuses on freshwater aquatic ecosystems. This report is the first detailed compilation ofstudies.conducted under the AES Action on Water Program. It covers work done from 1991 to 1994. Many studies have been concluded, while others are ongoing. Although data may not be available for all studies, or results are preliminary at this time, this report presents detailed background, objectives and methodology. -

Northern Athapaskan Conference, V2
NATIONAL MUSEUM MUSÉE NATIONAL OF MAN DE L’HOMME MERCURY SERIES COLLECTION MERCURE CANADIAN ETHNOLOGY SERVICE LE SERVICE CANADIEN D’ETHNOLOGIE PAPER No.27 DOSSIER No. 27 PROCEEDINGS: NORTHERN ATHAPASKAN CONFERENCE, 1971 VOLUME TWO EDITED BY A.McFADYEN CLARK NATIONAL MUSEUMS OF CANADA MUSÉES NATIONAUX DU CANADA OTTAWA 1975 BOARD OF TRUSTEES MUSEES NATIONAUX DU CANADA NATIONAL MUSEUMS OF CANADA CONSEIL D'ADMINISTRATION Mr. George Ignatieff Chairman M. André Bachand Vice-Président Dr. W.E. Beckel Member M. Jean des Gagniers Membre Mr. William Dodge Member M. André Fortier Membre Mr. R.H. Kroft Member Mme Marie-Paule LaBrëque Membre Mr. J.R. Longstaffe Member Dr. B. Margaret Meagher Member Dr. William Schneider Member M. Léon Simard Membre Mme Marie Tellier Membre Dr. Sally Weaver Member SECRETARY GENERAL SECRETAIRE GENERAL Mr. Bernard Ostry DIRECTOR DIRECTEUR NATIONAL MUSEUM OF MAN MUSEE NATIONAL DE L ’HOMME Dr. William E. Taylor, Jr. CHIEF CHEF CANADIAN ETHNOLOGY SERVICE SERVICE CANADIEN D'ETHNOLOGIE Dr. Barrie Reynolds Crown Copyright Reserved Droits réservés au nom de la Couronne NATIONAL MUSEUM MUSÉE NATIONAL OF MAN DE L’HOMME MERCURY SERIES COLLECTION MERCURE ISSN 0316-1854 CANADIAN ETHNOLOGY SERVICE LE SERVICE CANADIEN D'ETHNOLOGIE PAPER NO.27 DOSSIER NO. 27 ISSN 0316-1862 PROCEEDINGS: NORTHERN ATHAPASKAN CONFERENCE, 1971 VOLUME TWO EDITED BY A. McFADYEN CLARK Cover Illustration: Contact traditional Kutchin camp based on a drawing from: "Journal du Yukon 1847-48" by Alexander Hunter Murray, Ottawa 1910, p. 86. NATIONAL MUSEUMS OF CANADA MUSÉES NATIONAUX DU CANADA OTTAWA 1975 OBJECT OF THE MERCURY SERIES The Mercury Series is a publication of the National Museum of Man, National Museums of Canada, designed to permit the rapid dissemination of information pertaining to those disciplines for which the National Museum of Man is responsible. -

Consolidation of Wildlife Management Units
WILDLIFE ACT LOI SUR LA FAUNE CONSOLIDATION OF WILDLIFE CODIFICATION ADMINISTRATIVE MANAGEMENT UNITS DU RÈGLEMENT SUR LES REGULATIONS SECTEURS DE GESTION DE LA R.R.N.W.T. 1990,c.W-15 FAUNE R.R.T.N.-O. 1990, ch. W-15 AS AMENDED BY MODIFIÉ PAR R-091-93 R-091-93 R-020-96 R-020-96 R-104-98 (CIF 98/08/01) R-104-98 (EEV1998-08-01) This consolidation is not an official statement of the La presénte codification administrative ne constitue law. It is an office consolidation prepared for pas le texte officiel de la loi; elle n’est établie qu'à convenience of reference only. The authoritative text titre documentaire. Seuls les règlements contenus of regulations can be ascertained from the Revised dans les Règlements révisés des Territoires du Nord- Regulations of the Northwest Territories, 1990 and Ouest (1990) et dans les parutions mensuelles de la the monthly publication of Part II of the Northwest Partie II de la Gazette des Territoires du Nord-Ouest Territories Gazette (for regulations made before (dans le cas des règlements pris avant le 1 er avril April 1, 1999) and Part II of the Nunavut Gazette (for 1999) et de la Partie II de la Gazette du Nunavut regulations made on or after April 1, 1999). (dans le cas des règlements pris depuis le 1 er avril 1999) ont force de loi. WILDLIFE ACT LOI SUR LA FAUNE WILDLIFE MANAGEMENT UNITS RÈGLEMENT SUR LES SECTEURS REGULATIONS DE GESTION DE LA FAUNE 1. The wildlife management units shall be delimited 1. -

Nt73-36 Report.Pdf
TERRAIN EVALUATION WITH RESPECT T O PIPELINE CONSTRUCTION, MACKENZIE TRANSPORTATION CORRIDO R Southern Part, Lat . 60 0 to 64°N . b y N . W . Rutter, A . N . Boydell, K . W . Savigny Terrain Sciences Divisio n Geological Survey of Canad a Department of Energy, Mine s and Resource s an d R . O . van Everdinge n Hydrology Research Divisio n Inland Waters Directorat e Department of the Environmen t for th e Environmental-Social Progra m Northern Pipeline s December 197 3 Environmental-Social Committee Information Canad a Northern Pipelines, Cat . No . R72-1037 3 Task Force on Northern Oil Developmen t Report No . 73-36 QS-1532-000-EE-Al The data for this report were obtained as a resul t of investigations carried out under the Environmental - Social Program, Northern Pipelines, of the Task Forc e on Northern Oil Development, Government of Canada . While the studies and investigations were initiated t o provide information necessary for the assessment o f pipeline proposals, the knowledge gained is equall y useful in planning and assessing highways and othe r development projects . - 1 - TERRAIN EVALUATION WITH RESPECT TO PIPELINE CONSTRUCTION , MACKENZIE TRANSPORTATION CORRIDOR Southern Part, Lat . 60° to 64 ° by N . W . Rutter, A . N . Boydell, K . W . Savigny and R . O . van Everdinge n Table of Contents Page 1. SUMMARY 1 2. INTRODUCTION 2 3. CURRENT STATE OF KNOWLEDGE 2 4. STUDY AREA 4 5. METHODS AND SOURCES OF DATA 6 6. GLACIAL HISTORY g 7. RESULTS 10 7 .1 Area I - Southern Region 1 0 7 .1 .1 Overview 1 0 7 .1 .2 Surficial Deposits 1 -

Canada Topographical
University of Waikato Library: Map Collection Canada: topographical maps 1: 250,000 The Map Collection of the University of Waikato Library contains a comprehensive collection of maps from around the world with detailed coverage of New Zealand and the Pacific : Editions are first unless stated. These maps are held in storage on Level 1 Please ask a librarian if you would like to use one: Coverage of Canadian Provinces Province Covered by sectors On pages Alberta 72-74 and 82-84 pp. 14, 16 British Columbia 82-83, 92-94, 102-104 and 114 pp. 16-20 Manitoba 52-54 and 62-64 pp. 10, 12 New Brunswick 21 and 22 p. 3 Newfoundland and Labrador 01-02, 11, 13-14 and 23-25) pp. 1-4 Northwest Territories 65-66, 75-79, 85-89, 95-99 and 105-107) pp. 12-21 Nova Scotia 11 and 20-210) pp. 2-3 Nunavut 15-16, 25-27, 29, 35-39, 45-49, 55-59, 65-69, 76-79, pp. 3-7, 9-13, 86-87, 120, 340 and 560 15, 21 Ontario 30-32, 40-44 and 52-54 pp. 5, 6, 8-10 Prince Edward Island 11 and 21 p. 2 Quebec 11-14, 21-25 and 31-35 pp. 2-7 Saskatchewan 62-63 and 72-74 pp. 12, 14 Yukon 95,105-106 and 115-117 pp. 18, 20-21 The sector numbers begin in the southeast of Canada: They proceed west and north. 001 Newfoundland 001K Trepassey 3rd ed. 1989 001L St: Lawrence 4th ed. 1989 001M Belleoram 3rd ed.