Membrane Processes for the Dehydration of Organic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Development of a Process for N-Butanol Recovery from Abe
937 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 74, 2019 The Italian Association of Chemical Engineering Online at www.cetjournal.it Guest Editors: Sauro Pierucci, Jiří Jaromír Klemeš, Laura Piazza Copyright © 2019, AIDIC Servizi S.r.l. ISBN 978-88-95608-71-6; ISSN 2283-9216 DOI: 10.3303/CET1974157 Development of a Process for N-Butanol Recovery from Abe Wastewater Streams by Membrane Technology * Marco Stoller , Marco Bravi, Paola Russo, Roberto Bubbico, Barbara Mazzarotta Sapienza University of Rome, Dept. of Chemical Materials Environmental Engineering, Via Eudossiana 18, 00184 Rome, Italy [email protected] The aceton-butyl-ethanolic fermentation process (ABE) is a biotechnological process that leads to the production of acetone, n-butanol and ethanol (ABE compounds) from glucose sources and amides by use of certain biomasses. The process was developed initially during the middle of the last century and suffers from decline due to the greater petrochemical production of products and the lowering of the costs of the sector. Nowadays, the ABE process is regaining great interest because the fraction with the highest concentration, i.e. n-butanol, is an excellent constituent for biofuels. The ABE process has been optimized over time to obtain maximum yields of n-butanol, but the problem of separating and concentrating the butanol in the outlet stream of the ABE process persists. To allow an adequate use, often distillation by use of more columns is required. Moreover, the contained biomasses and suspended solids, in high quantity, must be eliminated, leading to overall high treatment costs. This work will report the main idea and some preliminary experimental results for the development and application of a process based on membrane technologies, to separate and concentrate the butanol from ABE process streams to sensibly reduce the difficulty to perform a final distillation. -

Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1969 Evaluation of Azeotropic Dehydration for the Preservation of Shrimp. James Edward Rutledge Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rutledge, James Edward, "Evaluation of Azeotropic Dehydration for the Preservation of Shrimp." (1969). LSU Historical Dissertations and Theses. 1689. https://digitalcommons.lsu.edu/gradschool_disstheses/1689 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been 70-9089 microfilmed exactly as received RUTLEDGE, James Edward, 1941- EVALUATION OF AZEOTROPIC DEHYDRATION FOR THE PRESERVATION OF SHRIMP. The Louisiana State University and Agricultural and Mechanical College, PhJD., 1969 Food Technology University Microfilms, Inc., Ann Arbor, Michigan Evaluation of Azeotropic Dehydration for the Preservation of Shrimp A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Food Science and Technology by James Edward Rutledge B.S., Texas A&M University, 1963 M.S., Texas A&M University, 1966 August, 1969 ACKNOWLEDGMENT The author wishes to express his sincere appreciation to his major professor, Dr, Fred H. Hoskins, for the guidance which he supplied not only in respect to this dissertation but also in regard to the author’s graduate career at Louisiana State University, Gratitude is also extended to Dr. -
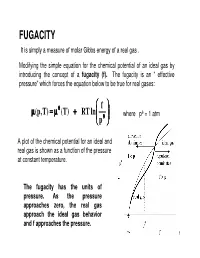
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Crystallization of Oxytetracycline from Fermentation Waste Liquor: Influence of Biopolymer Impurities
Journal of Colloid and Interface Science 279 (2004) 100–108 www.elsevier.com/locate/jcis Crystallization of oxytetracycline from fermentation waste liquor: influence of biopolymer impurities Shi-zhong Li a,1, Xiao-yan Li a,∗, Dianzuo Wang b a Environmental Engineering Research Centre, Department of Civil Engineering, The University of Hong Kong, Hong Kong, China b Chinese Academy of Engineering, Beijing 100038, China Received 28 January 2004; accepted 17 June 2004 Available online 29 July 2004 Abstract Organic impurities in the fermentation broth of antibiotic production impose great difficulties in the crystallization and recovery of antibi- otics from the concentrated waste liquor. In the present laboratory study, the inhibitory effect of biopolymers on antibiotic crystallization was investigated using oxytetracycline (OTC) as the model antibiotic. Organic impurities separated from actual OTC fermentation waste liquor by ultrafiltration were dosed into a pure OTC solution at various concentrations. The results demonstrated that small organic molecules with an apparent molecular weight (AMW) of below 10,000 Da did not affect OTC crystallization significantly. However, large biopolymers, especially polysaccharides, in the fermentation waste caused severe retardation of crystal growth and considerable deterioration in the pu- rity of the OTC crystallized. Atomic force microscopy (AFM) revealed that OTC nuclei formed in the solution attached to the surfaces of large organic molecules, probably polysaccharides, instead of being surrounded by proteins as previously thought. It is proposed that the attachment of OTC nuclei to biopolymers would prevent OTC from rapid crystallization, resulting in a high OTC residue in the aqueous phase. In addition, the adsorption of OTC clusters onto biopolymers would destabilize the colloidal system of organic macromolecules and promote particle flocculation. -

Review and Cost Analysis of Reverse Osmosis and Ultrafiltration
Review and cost analysis of reverse osmosis and ultrafiltration A Senior Project presented to Dr. Bruce Golden of Dairy Science California Polytechnic State University, San Luis Obispo In Partial Fulfillment of the Requirements for the Degree Bachelor of Dairy Science by Jared De Groot March, 2013 © 2013 Abstract The aim in this study was to determine whether implementing an on farm ultrafiltration system was profitable. Ultrafiltration was researched to reduce the amount of milk hauled from producer to processor. An ultrafiltration system involved milk that flowed through a semi-permeable membrane. Through this process water and small amounts of calcium and ash were pressured through the semi-permeable membrane. This permeate would then be able to feed heifers. The protein, fat, solids nonfat, and small amounts of calcium and ash were retained by the membrane. Through the process of ultrafiltration, raw milk was concentrated to three times its original concentration. With the use of ultrafiltration, the dairyman would need to one load of ultrafiltration milk instead of three loads of raw milk. The reduction in the cost of milk hauled and the feed presented to the heifers were the advantages in ultrafiltration. With the saved money on hauling and feeding of heifers, the initial costs of the system and the annual maintenance cost of the system exceed the benefits if implemented on De Groot Dairies. For the on farm ultrafiltration system to break even in ten years, the producer would need to be paid an additional $1.05 /cwt of retentate. This price included the reduced hauling cost and the money saved on the heifer ration. -

Sartorius Ultrafiltration and Protein Purification Products Brochure
Ultrafiltration & Protein Purification Products Fisher Scientific Contents General Information General Information Protein Purification Major Uses for Ultrafiltration 4 Vivapure® Ion Exchange Protein Purification Products 52 Process Alternatives 5 Vivawell Vac Vacuum Manifold Membrane Characteristics 6 Systems 55 Membrane Selection 7 Vivawell Vac 8-strip plate 57 Protein Concentration Vivapure® mini & maxiprep Purification Kits 59 Centrifugal Filtration Protein Concentration Vivapure® mini|maxiprep Protein Vivaspin 500 8 A & G Spin Columns 60 24-Well Ultrafiltration Frame 10 Vivapure® mini|maxiprep MC Spin Vivaspin 2 12 Columns 62 Centrisart I 15 Vivaspin 4 18 Vivapure Anti-HSA/IgG Kits 64 Vivaspin 6 20 Vivapure C18 65 Vivaspin 15 22 Vivaspin 15R 24 Virus Purification and Concentration Vivaspin 20 26 Vivaclear 29 Vivapure® Virus Purification and Concentration Kits 66 Pressure-Fugation DNA Concentration Vivacell 70 30 Adenovirus Purification with Vivacell 100 33 AdenoPACK Kits 67 Vivapure® AdenoPACK 20 68 Gas Pressure Filtration Vivapure® AdenoPACK 100 69 Vivacell 250 36 Vivapure® AdenoPACK 500 71 Tangential Flow Filtration Lentivirus Purification with Vivaflow 50 38 LentiSELECT Kit 73 Vivaflow 200 40 Vivapure® LentiSELECT 40 74 Solvent Adsorption Vivapore 2, 5, 10|20 43 Vivapure® LentiSELECT 500 75 Protein Purification Ultrafiltration Membrane Discs 45 Vivapure® LentiSELECT 1000 76 DNA Concentration Application Notes 77 Vivacon® 500 47 1. Desalting and Buffer Exchange with Vivaspin Centrifugal Concentrators 79 Vivacon® 250 2. Treatment -

By a 965% ATT'ys
July 19, 1960 A. WATZL ETAL 2,945,788 PROCESS FOR THE PURIFICATION OF DIMETHYLTEREPHTHALATE Filed Nov. 19, l956 AZEOTROPE DISTILLATE CONDENSER CONDENSED WACUUM DISTILLATE DRY DISTILLATION MXTURE N2 COLUMN MPURE - SOD WACUUMFILTER ETHYLENE DMETHYL DMETHYL GLYCOL TEREPHTHALATE TEREPHTHALATE ETHYLENE GLYCOL INVENTORS: ANTON WATZL by aERHARD 965% SIGGEL ATT'YS 2,945,788 Patented July 19, 1960 2 phthalate is immediately suitable for reesterification or Subsequent polycondensation to polyethylene terephthal 2,945,788 ate. There are gained polycondensates of high degree of PROCESS FOR THE PURIFICATION OF viscosity with K values from 50 to 57. DMETHYLTEREPHTHALATE The best mode contemplated for practicing the inven Anton Watz, Kleinwallstadt (Ufr), and Erhard Siggel, tion involves the use of ethylene glycol as the aliphatic Laudenbach (Main), Germany, assignors to Vereinigte glycol. The following is a specific illustration thereof. Glanzstoff-Fabriken A.G., Wuppertal-Elberfeld, Ger Example many Thirty grams of crude dimethylterephthalate are mixed Filed Nov.19, 1956, Ser. No. 622,778 in a flask with 270 grams of ethylene glycol and azeo tropically distilled with the introduction of dry nitrogen 4 Claims. (C. 202-42) in a column of 30 cm. at about 44 Torr (1 Torr equals 1 mm. Hg). The azeotrope goes over at about 120° C. i This invention, in general, relates to production of into a cooled condenser. The dimethylterephthalate dimethylterephthalate and more particularly to the puri 5 separated from the glycol by vacuum filtering can be fication thereof. used immediately for reesterification or for polyconden The purification of dimethylterephthalate can be car sation. This process is illustrated in the flow sheet of ried out either by distillation or by recrystallization from the accompanying drawing. -

Heterogeneous Azeotropic Distillation
PROSIMPLUS APPLICATION EXAMPLE HETEROGENEOUS AZEOTROPIC DISTILLATION EXAMPLE PURPOSE This example illustrates a high purity separation process of an azeotropic mixture (ethanol-water) through heterogeneous azeotropic distillation. This process includes distillation columns. Additionally these rigorous multi- stage separation modules are part of a recycling stream, demonstrating the efficiency of ProSimPlus convergence methods. Specifications are set on output streams in order to insure the required purity. This example illustrates the way to set "non-standard" specifications in the multi-stage separation modules. Three phase calculations (vapor-liquid- liquid) are done with the taken into account of possible liquid phase splitting. ACCESS Free-Internet Restricted to ProSim clients Restricted Confidential CORRESPONDING PROSIMPLUS FILE PSPS_EX_EN-Heterogeneous-Azeotropic-Distillation.pmp3 . Reader is reminded that this use case is only an example and should not be used for other purposes. Although this example is based on actual case it may not be considered as typical nor are the data used always the most accurate available. ProSim shall have no responsibility or liability for damages arising out of or related to the use of the results of calculations based on this example. Copyright © 2009 ProSim, Labège, France - All rights reserved www.prosim.net Heterogeneous azeotropic distillation Version: March, 2009 Page: 2 / 12 TABLE OF CONTENTS 1. PROCESS MODELING 3 1.1. Process description 3 1.2. Process flowsheet 5 1.3. Specifications 6 1.4. Components 6 1.5. Thermodynamic model 7 1.6. Operating conditions 7 1.7. "Hints and Tips" 9 2. RESULTS 9 2.1. Comments on results 9 2.2. Mass and energy balances 10 2.3. -

Enhanced Distillation • Use of Triangular Graphs
Lecture 15. Enhanced Distillation and Supercritical Extraction (1) [Ch. 11] • Enhanced Distillation • Use of Triangular Graphs - Zeotropic mixture -Mixture forming one minimum-boiling azeotrope - Mixture forming two minimum-boiling azeotropes • Residue-Curve Maps • Distillation-Curve Maps • Product-Composition Regions at Total Reflux Enhanced Distillation • Cases when ordinary distillation is not economical - Boiling point difference between components is less than 50℃ - Relative volatility is less than 1.10 - Mixture forms an azeotrope • Enhanced distillation - Extractive distillation : adding a large amount of solvent - Salt distillation : adding a soluble , ionic salt - Pressure-swing distillation : operating at two different pressures - Homogeneous azeotropic distillation : adding an entrainer - Heterogeneous azeotropic distillation : adding an entrainer - Reactive distillation : adding a separating agent to react selectively and reversibly with feed component(s) Zeotropic vs. Azeotropic Systems Binary zeotropic system Binary azeotropic system P = constant AtAzeotrope C T-y T-x Pure B xA, yA Pure A Use of Triangular Graphs • Triangular vapor-liquid diagram for ternary mixture - Too complex to understand • Plot with only equilibr ium liquid composition fofoatenayr a ternary mixture - Easy to understand -Convenient to use - Provide useful information in distillation of ternary components . Residue curve . Distill ati on curve Distillation Curves for Ternary Systems Each curve in each diagram is the locus of possible equilibrium liquid-phase -

S-Layer Ultrafiltration Membranes
membranes Review S-Layer Ultrafiltration Membranes Bernhard Schuster * and Uwe B. Sleytr * Institute for Synthetic Bioarchitectures, Department of NanoBiotechnology, BOKU—University of Natural Resources and Life Sciences, Vienna, Muthgasse 11, 1190 Vienna, Austria * Correspondence: [email protected] (B.S.); [email protected] (U.B.S.) Abstract: Monomolecular arrays of protein subunits forming surface layers (S-layers) are the most common outermost cell envelope components of prokaryotic organisms (bacteria and archaea). Since S-layers are periodic structures, they exhibit identical physicochemical properties for each constituent molecular unit down to the sub-nanometer level. Pores passing through S-layers show identical size and morphology and are in the range of ultrafiltration membranes. The functional groups on the surface and in the pores of the S-layer protein lattice are accessible for chemical modifications and for binding functional molecules in very precise fashion. S-layer ultrafiltration membranes (SUMs) can be produced by depositing S-layer fragments as a coherent (multi)layer on microfiltration membranes. After inter- and intramolecular crosslinking of the composite structure, the chemical and thermal resistance of these membranes was shown to be comparable to polyamide membranes. Chemical modification and/or specific binding of differently sized molecules allow the tuning of the surface properties and molecular sieving characteristics of SUMs. SUMs can be utilized as matrices for the controlled immobilization of functional biomolecules (e.g., ligands, enzymes, antibodies, and antigens) as required for many applications (e.g., biosensors, diagnostics, enzyme- and affinity- membranes). Finally, SUM represent unique supporting structures for stabilizing functional lipid membranes at meso- and macroscopic scale. -

Membrane Filtration
Membrane Filtration A membrane is a thin layer of semi-permeable material that separates substances when a driving force is applied across the membrane. Membrane processes are increasingly used for removal of bacteria, microorganisms, particulates, and natural organic material, which can impart color, tastes, and odors to water and react with disinfectants to form disinfection byproducts. As advancements are made in membrane production and module design, capital and operating costs continue to decline. The membrane processes discussed here are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). MICROFILTRATION Microfiltration is loosely defined as a membrane separation process using membranes with a pore size of approximately 0.03 to 10 micronas (1 micron = 0.0001 millimeter), a molecular weight cut-off (MWCO) of greater than 1000,000 daltons and a relatively low feed water operating pressure of approximately 100 to 400 kPa (15 to 60psi) Materials removed by MF include sand, silt, clays, Giardia lamblia and Crypotosporidium cysts, algae, and some bacterial species. MF is not an absolute barrier to viruses. However, when used in combination with disinfection, MF appears to control these microorganisms in water. There is a growing emphasis on limiting the concentrations and number of chemicals that are applied during water treatment. By physically removing the pathogens, membrane filtration can significantly reduce chemical addition, such as chlorination. Another application for the technology is for removal of natural synthetic organic matter to reduce fouling potential. In its normal operation, MF removes little or no organic matter; however, when pretreatment is applied, increased removal of organic material can occur.