Provisional Peer-Reviewed Toxicity Values for Methylcyclopentane (Casrn 96-37-7)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Information for “Oligomeric Models for Estimation of Polydimethylsiloxane
Electronic Supplementary Material (ESI) for Environmental Science: Processes & Impacts. This journal is © The Royal Society of Chemistry 2016 Supplementary Information for “Oligomeric models for estimation of polydimethylsiloxane- water partition ratios with COSMO-RS theory: Impact of the combinatorial term on absolute error” by J. Mark Parnis and Donald Mackay. Contents: 1) The complete set of chemicals, with the published values of log K(PDMS-w), and the abbreviated reference, corresponding to references given in the principle text. 1) The complete set of chemicals, with the published values of log KPDMS-w. Hsieh 2011 CHAO 2014 PCB18 4.91 PCB203 7.09 PCB16 5.12 PCB195 6.89 PCB32 5.12 PCB194 6.79 PCB28,31 5.17 Benzyl alcohol -0.35 PCB33,53 5.18 4-Fluorophenol -0.28 PCB22 5.30 m-Cresol -0.03 PCB52 5.48 Phenethyl alcohol 0.12 PCB47,48 5.49 3-Methylbenzyl alcohol 0.17 PCB44 5.44 3-Chlorophenol 0.31 PCB71 5.49 3,5-Dimethylphenol 0.42 PCB41 5.49 3-Bromophenol 0.46 PCB70 5.79 4-Ethylphenol 0.60 PCB66 5.70 4-Chloroaniline 0.84 PCB95 5.77 Phenyl acetate 0.86 PCB56,60 5.86 Benzonitrile 1.04 PCB101 6.01 Acetophenone 1.04 PCB99 6.17 4-Chloroacetophenone 1.64 PCB83 6.02 Methyl benzoate 1.65 PCB97 6.05 Benzene 1.76 PCB87 6.19 Ethylbenzoate 2.12 PCB85 6.38 Toluene 2.28 PCB110 6.03 4-Chloroanisole 2.37 PCB151 6.31 Chlorobenzene 2.40 PCB135 6.48 Bromobenzene 2.51 PCB149 6.42 o-Xylene 2.69 PCB118 6.23 m-Xylene 2.73 PCB146 6.66 Iodobenzene 2.73 PCB153 6.62 p-Xylene 2.75 PCB105,132 6.40 Ethylbenzene 2.75 PCB179 6.73 Phenol -0.18 PCB141 6.73 Naphthalene 2.83 PCB138 6.61 Cyclopentane 2.85 PCB163 6.56 4-Chlorotoluene 2.87 PCB158 6.83 Methylcyclopentane 3.13 PCB187 6.96 Propylbenzene 3.14 PCB182 6.96 Isopropylbenzene 3.15 PCB183 6.26 1,3,5-Trimethylbenzene 3.16 PCB128 6.61 1-Methyl-4-Ethylbenzene 3.20 PCB185 6.86 Cyclohexane 3.20 PCB174 7.04 1-Methylnaphthalene 3.26 PCB177 7.02 1,2,4-Trimethylbenzene 3.34 PCB171,202 6.78 tert-Butylbenzene 3.34 PCB180 6.89 Biphenyl 3.37 PCB170 6.82 2,3-Dimethylbutane 3.37 PCB201 7.06 2-Methylpentane 3.51 CHAO 2014 Kang et al. -

Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002
Minutes of the IUPAC Chemical Nomenclature and Structure Representation Division (VIII) Committee Meeting Boston, MA, USA, August 18, 2002 Members Present: Dr Stephen Heller, Prof Herbert Kaesz, Prof Dr Alexander Lawson, Prof G. Jeffrey Leigh, Dr Alan McNaught (President), Dr. Gerard Moss, Prof Bruce Novak, Dr Warren Powell (Secretary), Dr William Town, Dr Antony Williams Members Absent: Dr. Michael Dennis, Prof Michael Hess National representatives Present: Prof Roberto de Barros Faria (Brazil) The second meeting of the Division Committee of the IUPAC Division of Chemical Nomenclature and Structure Representation held in the Great Republic Room of the Westin Hotel in Boston, Massachusetts, USA was convened by President Alan McNaught at 9:00 a.m. on Sunday, August 18, 2002. 1.0 President McNaught welcomed the members to this meeting in Boston and offered a special welcome to the National Representative from Brazil, Prof Roberto de Barros Faria. He also noted that Dr Michael Dennis and Prof Michael Hess were unable to be with us. Each of the attendees introduced himself and provided a brief bit of background information. Housekeeping details regarding breaks and lunch were announced and an invitation to a reception from the U. S. National Committee for IUPAC on Tuesday, August 20 was noted. 2.0 The agenda as circulated was approved with the addition of a report from Dr Moss on the activity on his website. 3.0 The minutes of the Division Committee Meeting in Cambridge, UK, January 25, 2002 as posted on the Webboard (http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.rtf and http://www.rsc.org/IUPAC8/attachments/MinutesDivCommJan2002.pdf) were approved with the following corrections: 3.1 The name Dr Gerard Moss should be added to the members present listing. -

Combined PIANO Standard
Combined PIANO Standard Product #: VHG-PIANO-COM-0.1 Lot #: 711069047B Concentration Concentration Component Component (Wt.%) (Wt.%) Isoparaffins Isopentane 0.3371 4-Methylheptane 0.4939 2,3-Dimethylbutane 0.0691 3-Methylheptane 0.8406 2-Methylpentane 0.5031 3-Ethylhexane 0.1097 3-Methylpentane 0.8271 3,3-Dimethylheptane 0.2590 2,2-Dimethylpentane 0.2746 2,5-Dimethylheptane 0.8678 2,4-Dimethylpentane 0.5679 3,5-Dimethylheptane 0.1169 2,2,3-Trimethylbutane 0.6042 2,3-Dimethylheptane 0.2288 3,3-Dimethylpentane 0.2873 3,4-Dimethylheptane 0.5668 2-Methylhexane 0.3643 2-Methyloctane 0.5791 2,3-Dimethylpentane 0.2751 3-Methyloctane 0.8645 3-Methylhexane 0.2475 3,3-Diethylpentane 0.2424 3-Ethylpentane 0.0813 2,2-Dimethyloctane 0.5024 2,2-Dimethylhexane 0.2027 3,3-Dimethyloctane 0.4902 2,5-Dimethylhexane 0.5727 2,3-Dimethyloctane 0.5927 2,2,3-Trimethylpentane 0.2658 3-Ethyloctane 0.5694 2,4-Dimethylhexane 0.2542 2-Methylnonane 0.5727 2,3-Dimethylhexane 0.2492 3-Methylnonane 0.8889 2-Methylheptane 0.6744 Aromatics Benzene 1.6808 1-Methyl-3-n-propylbenzene 0.4972 Toluene 1.0772 1-Methyl-4-n-propylbenzene 0.5237 Ethylbenzene 1.5918 n-Butylbenzene 0.5198 m-Xylene 0.5341 1,2-Diethylbenzene 0.2577 p-Xylene 1.1328 1-Methyl-2-n-propylbenzene 0.5282 o-Xylene 0.5315 1,4-Dimethyl-2-ethylbenzene 0.5379 Isopropylbenzene 0.5235 1,3-Dimethyl-5-ethylbenzene 0.5176 n-Propylbenzene 1.0731 1,2-Dimethyl-4-ethylbenzene 0.5310 1-Methyl-3-ethylbenzene 0.5181 1,3-Dimethyl-2-ethylbenzene 0.2695 1-Methyl-4-ethylbenzene 0.5104 1,2-Dimethyl-3-ethylbenzene 0.5151 1,3,5-Trimethylbenzene -

G.J. Chemical Company, Inc. Safety Data Sheet
G.J. CHEMICAL COMPANY, INC. SAFETY DATA SHEET . PRODUCT IDENTIFIER 1.1 PRODUCT NAME:------------> Hexane (All Grades) MIXTURE OF ISOMERS PRODUCT NUMBER(S)----------> 175300, 175310 TRADE NAMES/SYNONYMS----> Mixture of n-Hexane and Hexane isomers CAS-No: 110-54-3 CHEMICAL FAMILY: Aliphatic Hydrocarbon 1.2 RELEVANT IDENTIFIED USES OF THE SUBSTANCE OR MIXTURE AND USES ADVISED AGAINST RECOMMENDED USE: Manufacture of substances. Laboratory chemicals. USES ADVISED AGAINST: No information available 1.3 DETAILS OF THE SUPPLIER OF THE SAFETY DATA SHEET Company: G.J. CHEMICAL CO., INC. Address: 40 VERONICA AVENUE SOMERSET, NJ 08873 Telephone: 1-973-589-1450 Fax: 1-973-589-3072 1.4 Emergency Telephone Number Emergency Phone: 1-800-424-9300 (CHEMTREC) . HAZARDS IDENTIFICATION 2.1 Classification of the substance or mixture GHS Classification in accordance with 29CFR 1910 (OSHA HCS) Flammable liquids (Category 2), H225 Skin irritation (Category 2), H315 Eye irritation (Category 2A), H319 Reproductive toxicity (Category 2), H361f Specific target organ toxicity - single exposure (Category 3), Central Nervous system, H336 Specific target organ toxicity - repeated exposure, Oral (Category 2), H373 Aspiration hazard (Category 1), H304 Acute aquatic toxicity (Category 2), H401 Chronic aquatic toxicity (Category 2), H411 2.2 GHS Label elements, including precautionary statements Pictogram GHS02 GHS08 GHS07 GHS09 Signal word: DANGER Hazard statement(s) H225 Highly flammable liquid and vapor. H304 May be fatal if swallowed and enters airways. H315 Causes skin irritation. H319 Causes serious eye irritation. H336 May cause drowsiness or dizziness. H361f Suspected of damaging fertility or the unborn child. H373 May cause damage to organs through prolonged or repeated exposure if swallowed. -

US and Canadian
Version: 1.0 Revision Date: 03/14/2018 SAFETY DATA SHEET 1. Identification Material name: TREMPLY TPO BONDING ADHESIVE 5 GL Material: 423300 805 Recommended use and restriction on use Recommended use : Adhesive Restrictions on use: Not known. Manufacturer/Importer/Supplier/Distributor Information Tremco U.S. Roofing 3735 Green Road Beachwood OH 44122 US Contact person : EH&S Department Telephone : 216-292-5000 Emergency telephone number : 1-800-424-9300 (US); 1-613-996-6666 (Canada) 2. Hazard(s) identification Hazard Classification Physical Hazards Flammable liquids Category 2 Health Hazards Skin Corrosion/Irritation Category 2 Serious Eye Damage/Eye Irritation Category 2A Germ Cell Mutagenicity Category 1B Carcinogenicity Category 1B Toxic to reproduction Category 2 Specific Target Organ Toxicity - Category 1 Repeated Exposure Unknown toxicity - Health Acute toxicity, oral 22.57 % Acute toxicity, dermal 22.72 % Acute toxicity, inhalation, vapor 37.75 % Acute toxicity, inhalation, dust 100 % or mist Environmental Hazards Acute hazards to the aquatic Category 3 environment Unknown toxicity - Environment 1/20 000000026448 Version: 1.0 Revision Date: 03/14/2018 Acute hazards to the aquatic 24.15 % environment Chronic hazards to the aquatic 100 % environment Label Elements Hazard Symbol : Signal Word: Danger Hazard Statement: Highly flammable liquid and vapour. Causes skin irritation. Causes serious eye irritation. May cause drowsiness or dizziness. Suspected of damaging fertility or the unborn child. Causes damage to organs through prolonged or repeated exposure if inhaled. Harmful to aquatic life. Precautionary Statements Prevention: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Keep container tightly closed. Ground and bond container and receiving equipment. -

Compilation and Analysis of Types and Concentrations of Airborne 3 Chemicals Measured in Various Indoor and Outdoor Human 4 Environments 5 6 7 8 9 1 2 10 J
Chemosphere,127,70-86,2015 1 2 Compilation and Analysis of Types and Concentrations of Airborne 3 Chemicals Measured in Various Indoor and Outdoor Human 4 Environments 5 6 7 8 9 1 2 10 J. Enrique Cometto-Muñiz and Michael H. Abraham 11 1 12 University of California, San Diego, La Jolla, California, USA and 2 13 Department of Chemistry, University College London, London, UK 14 15 16 17 18 19 20 Address for correspondence: 21 22 J. Enrique Cometto-Muñiz, Ph.D. 23 Research Scientist Emeritus, UCSD 24 8950 Villa La Jolla Drive, Suite C135 25 La Jolla, CA 92037 26 USA 27 28 29 Phone: (858) 622-5832 30 e-mail: [email protected] 31 32 33 34 35 36 37 38 39 40 Running head: Airborne Chemical Concentrations Indoors and Outdoors 41 1 41 Abstract 42 43 The main purpose of this article is to summarize and illustrate the results of a literature search on 44 the types, levels, relative concentrations, concentration spread of individual chemicals, and number of 45 airborne compounds (mostly volatile organic compounds, VOCs) that have been found, measured, and 46 reported both indoors and outdoors. Two broad categories of indoor environments are considered: 1) 47 Home/School, and 2) Commercial spaces. Also, two categories of outdoor environments are considered: 48 1) Non-industrial and 2) Industrial (the latter represented by the vicinity of a pig farm and the vicinity of an 49 oil refinery). The outcome is presented as a series of graphs and tables containing the following statistics: 50 geometric mean, arithmetic mean, median, standard deviation, variance, standard error, interquartile 51 distance, minimum value, maximum value, and number of data (data count) for the air concentration of 52 each reported compound in a given environment. -

Compound Other Saturated Aliphatic Hydrocarbons C6-C8 Data Collection Sheet (1/4)
Compound Other saturated aliphatic hydrocarbons C6-C8 Data collection sheet (1/4) See Annex 1 for existing human health-based CLP See Table 1 for CAS numbers in the factsheet classifications in the factsheet Organisation name AgBB ANSES BAuA ECHA ECHA DNEL(general population, long-term, inhalation, systemic) DNEL(general population, long-term, [Hydrocarbons, C6-C7, n- NIK (=LCI) CLI (=LCI) OEL (aliphatic C6-C8) inhalation, systemic) alkanes, isoalkanes, (octane & isooctane) cyclics, <5% n-hexane” Risk value name (EC number: 921-024-6)] Risk value (µg/m3) 15000 10000 700000 608000 608000 Risk value (ppb) N/A N/A N/A N/A ~130000 Reference period Chronic Chronic Chronic Chronic Chronic Year 2012 1993 2017 2011, updated 2017 2011, updated in 2017 GLP-guideline study GLP-guideline study (OECD guideline 413) as (OECD guideline 413) as Key study N/A N/A N/A reported in the reported in the registration dossiers for registration dossier octane and isooctane Subchronic inhalation Subchronic inhalation Study type N/A N/A N/A study study Species N/A N/A N/A Rat Rat 6 h/d, 5 d/wk for 13 6 h/d, 5 d/wk for 13 Duration of exposure N/A N/A N/A weeks weeks Value based on provisional The group limit value Subchronic toxicity or Subchronic toxicity or OEL by the German BAuA is based on the 700 neurotoxic effects of neurotoxic effects of Critical effect for C5-C8 aliphatic N/A mg/m³ (200 ppm) “light alkylate naphtha “light alkylate naphtha hydrocarbons in 2012 limit value for distillate” (CAS 64741-66- distillate” (CAS 64741-66- (BAuA, 2012). -

Health Effects Review of 2010 Ambient Air Network Monitoring
TCEQ Interoffice Memorandum To: Jaime Garza, Regional Director From: Sabine Lange, Ph.D. Toxicology Division, Office of the Executive Director Date: August 4, 2015 Subject: Toxicological Review of 2014 Ambient Air Network Monitoring Data in Region 15, Harlingen Conclusions All 24-hour average and annual average concentrations of 84 volatile organic compounds (VOCs), 16 polycyclic aromatic hydrocarbons (PAHs), and two metals measured in total suspended particulate matter (TSP) were below their respective Texas Commission on Environmental Quality (TCEQ) air monitoring comparison values (AMCVs) and would not be expected to cause adverse health effects or vegetation effects. Background Ambient air sampling conducted at two monitoring network sites in Region 15, Harlingen during 2014 was evaluated by the Toxicology Division (TD). Table 1 indicates the location and monitored compounds at two Community Air Toxics Monitoring Network sites in Region 15. Hyperlinks are provided in Table 1 for more detailed information on each monitoring site. The TD reviewed air monitoring summary results for VOCs, PAHs, and speciated metals data from 24-hour TSP samples collected every sixth-day. For a complete list of all examined chemicals, please see Lists 1, 2, and 3 in Attachment A. The TCEQ Monitoring Division reported the data for all chemicals evaluated in this memorandum. All data collected [84 VOCs, 16 PAHs, and 2 metals (TSP)] from the Brownsville and Mission monitoring sites met the data completeness objective of 75 percent data return. Twenty-four-hour air samples collected every sixth-day for a year are designed to provide representative long-term average concentrations. In order to be able to evaluate 24-hour monitoring data more fully, TCEQ has developed 24-hour AMCVs for specific chemicals. -

Identification of Polycyclic Aromatic Hydrocarbons in the Supercritical
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2008 Identification of Polycyclic Aromatic Hydrocarbons in the Supercritical Pyrolysis Products of Synthetic Jet Fuel S-8 and Methylcyclohexane Jorge Oswaldo Ona Ruales Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Chemical Engineering Commons Recommended Citation Ona Ruales, Jorge Oswaldo, "Identification of Polycyclic Aromatic Hydrocarbons in the Supercritical Pyrolysis Products of Synthetic Jet Fuel S-8 and Methylcyclohexane" (2008). LSU Doctoral Dissertations. 2092. https://digitalcommons.lsu.edu/gradschool_dissertations/2092 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. IDENTIFICATION OF POLYCYCLIC AROMATIC HYDROCARBONS IN THE SUPERCRITICAL PYROLYSIS PRODUCTS OF SYNTHETIC JET FUEL S-8 AND METHYLCYCLOHEXANE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemical Engineering by Jorge Oswaldo Oña Ruales Ingeniero Quimico, Universidad Central del Ecuador – Quito, 2000 May, 2008 ACKNOWLEDGEMENTS I would like to thank the following: My advisor, Prof. Mary Julia Wornat, for her wise guidance during the last six years, for introduce me to the world of polycyclic aromatic hydrocarbons, and for showing me how to transform my observations into words and the words into scientific papers. -

Metals Compounds Carbonyls Compounds Polycyclic Aromatic Hydrocarbons (PAH)
Metals Compounds ANTIMONY(TSP) ARSENIC(TSP) BERRYLIUM(TSP) CADMIUM(TSP) CHROMIUM(TSP) COBALT(TSP) LEAD(TSP) MANGANESE(TSP) NICKEL(TSP) SELENIUM(TSP) ZINC(TSP) Carbonyls Compounds ACETALDEHYDE ACETONE ACROLEIN BENZALDEHYDE BUTYRALDEHYDE FORMALDEHYDE PROPIONALDEHYDE Polycyclic Aromatic Hydrocarbons (PAH) NAPHTHALENE ACENAPHTHYLENE(TSP) ACENAPHTHENE(TSP) FLUORENE(TSP) PHENANTHRENE ANTHRACENE FLUORANTHENE(TSP) PYRENE(TSP) BENZO(A)ANTHRACENE(TSP) CHRYSENE(TSP) BENZO(E)PYRENE(TSP) BENZO(B)FLUORANTHENE BENZO(K)FLUORANTHENE BENZO(A)PYRENE(TSP) DIBENZO(A-H)ANTHRACENE BENZO(g,h,i)PERYLENE(TSP) INDENO(1-2-3-cd)PYRENE Volatile Organic Compounds (VOC) 1-1-2-2-Tetrachloroethane 1-1-2-Trichloroethane 1-1-DiChloroEthane 1-1-DiChloroEthylene 1-2-4-TriChloroBenzene 1-2-4-TriMethylBenzene 1-2-DiChloroBenzene 1-2-DiChloroPropane 1-3-5-TriMethylBenzene 1-3-Butadiene 1-3-DichloroBenzene 1-4-DichloroBenzene 4-EthylToluene Benzene BenzylChloride Bromomethane Carbon Tetrachloride Chlorobenzene Chloroethane Chloroform Chloromethane cis-1-2-DiChloroEthene cis-1-3-DiChloroPropylene Cyclohexane DiChloroDiFluoroMethane DiChloroMethane(Methylene Chloride) Ethylbenzene Ethylene DiBromide(1,2-Dibromoethane) Ethylene DiChloride(1,2-Dichlororethane) Freon 113 Freon 114 HexaChloroButadiene M/P Xylene (m & p-Dimethylbenzene) MethylChloroform(1,1,1-Trichloroethane) O-Xylene (o-Dimethylbenzene) Styrene TetraChloroEthylene Toluene Trans-1-3-DiChloroPropylene TriChloroEthylene TriChloroFluoroMethane(Freon 11) Vinyl Chloride(Chloroethene) Photochemical Assessment Monitoring (PAMS) -

Introduction to Organic Chemistry: Hydrocarbons
Chapter 12 – Introduction to Organic Chemistry: Hydrocarbons Section 12.1 – Alkanes Goal: Identify properties characteristic of organic or inorganic compounds. Summary: Organic compounds: always contain carbon and hydrogen (thought sometimes other nonmetals as well.) Inorganic compounds: all other compounds. Often ionic (metal + nonmetal) Organic compounds Inorganic compounds Covalent bonds Often ionic or contain polar covalent bonds Most form nonpolar molecules Usually soluble in water Low melting and boiling points High melting and boiling points Not very soluble in water Most are soluble, unless nonpolar Dissolve as molecules in solutions (not ions) Produce ions in water Burn vigorously in air Do not burn in air Each carbon atom covalently bonds 4 times (due to its 4 valence electrons). When 4 individual groups are attached to a carbon, it has a tetrahedral geometry. Practice Problems 1. Identify each the following as a formula of an organic or inorganic compound: a. KCl b. C4H10 c. C2H6O d. H2SO4 e. CaCl2 f. C3H7Cl 2. Identify each of the following as a formula of an organic or inorganic compound: a. C6H12O6 b. K3PO4 c. I2 d. C2H6S e. C10H22 f. C4H9Br 3. Identify each of the following properties as more typical of an organic or inorganic compound: a. is soluble in water b. has a low boiling point c. contains carbon and hydrogen d. contains ionic bonds 4. Identify each of the following properties as more typical of an organic or inorganic compound: a. contains Li and F b. is a gas at room temperature c. contains covalent bonds d. produces ions in water 5. -
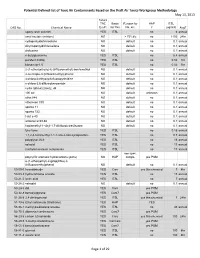
Potential Defined List of Toxic Air Contaminants Based on the Draft Air Toxics Workgroup Methodology May 13, 2013 Future TAC Basis Reason for HAP ITSL CAS No
Potential Defined List of Toxic Air Contaminants Based on the Draft Air Toxics Workgroup Methodology May 13, 2013 Future TAC Basis Reason for HAP ITSL CAS No. Chemical Name Ex.#1 for Yes No, etc. ? (ug/m3) AvgT epoxy resin solution YES ITSL no 6 annual amyl acetate (mixture) NO > 75%tile no 1100 24hr cyclopentyldichlorosilane NO default no 0.1 annual dicyclopentyldichlorosilane NO default no 0.1 annual disiloxane NO default no 0.1 annual n-butylglucamine YES ITSL no 6.4 annual purafect 4000g YES ITSL no 0.02 1hr biosam tp-1.5 YES ITSL no 0.02 1hr 2-(1-ethoxyethoxy)-6-(trifluoromethyl)-benzenethiol NO default no 0.1 annual 2-mercapto-3-(trifluoromethyl)-phenol NO default no 0.1 annual 4-chloro-2-ethyoxy-6-fluoropyrimidine NO default no 0.1 annual n-chloro-2,6-difluorobenzamide NO default no 0.1 annual cyclic (phme)2(me)2, d4 NO default no 0.1 annual 100 sxl NO default unknown 0.1 annual atlox 848 NO default no 0.1 annual ethomeen t/30 NO default no 0.1 annual sponto 11 NO default no 0.1 annual sponto 723 NO default no 0.1 annual t-det c-40 NO default no 0.1 annual witconol al 69-66 NO default no 0.1 annual heptamethyl-1-vinyl-1,7-dichlorotetrasilazane NO default no 0.1 annual fyre-zyme YES ITSL no 0.15 annual 1,1,2,4-tetramethyl-1-1-1-sila-2-aza-cyclopentane YES ITSL no 0.7 annual polyglycol 26-3 YES ITSL no 16 annual ad acid YES ITSL no 17 annual triethylammonium suleptanate YES ITSL no 17 annual see spec.