Sexual Behaviour of Rams:Male Orientation and Its Endocrine Correlates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caesarean Section Or Vaginal Delivery in the 21St Century
CAESAREAN SECTION OR VAGINAL DELIVERY IN THE 21ST CENTURY ntil the 20th Century, caesarean fluid embolism. The absolute risk of trans-placentally to the foetus, prepar- section (C/S) was a feared op- death with C/S in high and middle- ing the foetus to adopt its mother’s Ueration. The ubiquitous classical resource settings is between 1/2000 and microbiome. C/S interferes with neonatal uterine incision meant high maternal 1/4000 (2, 3). In subsequent pregnancies, exposure to maternal vaginal and skin mortality from bleeding and future the risk of placenta previa, placenta flora, leading to colonization with other uterine rupture. Even with aseptic surgi- accreta and uterine rupture is increased. environmental microbes and an altered cal technique, sepsis was common and These conditions increase maternal microbiome. Routine antibiotic exposure lethal without antibiotics. The operation mortality and severe maternal morbid- with C/S likely alters this further. was used almost solely to save the life of ity cumulatively with each subsequent Microbial exposure and the stress of a mother in whom vaginal delivery was C/S. This is of particular importance to labour also lead to marked activation extremely dangerous, such as one with women having large families. of immune system markers in the cord placenta previa. Foetal death and the use blood of neonates born vaginally or by of intrauterine foetal destructive proce- Maternal Benefits C/S after labour. These changes are absent dures, which carry their own morbidity, C/S has a modest protective effect against in the cord blood of neonates born by were often preferable to C/S. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Highlights of the Museum of Zoology Highlights on the Blue Route
Highlights of the Museum of Zoology Highlights on the Blue Route Ray-finned Fishes 1 The ray-finned fishes are the most diverse group of backboned animals alive today. From the air-breathing Polypterus with its bony scales to the inflated porcupine fish covered in spines; fish that hear by picking up sounds with the swim bladder and transferring them to the ear along a series of bones to the electrosense of mormyrids; the long fins of flying fish helping them to glide above the ocean surface to the amazing camouflage of the leafy seadragon… the range of adaptations seen in these animals is extraordinary. The origin of limbs 2 The work of Prof Jenny Clack (1947-2020) and her team here at the Museum has revolutionised our understanding of the origin of limbs in vertebrates. Her work on the Devonian tetrapods Acanthostega and Ichthyostega showed that they had eight fingers and seven toes respectively on their paddle-like limbs. These animals also had functional gills and other features that suggest that they were aquatic. More recent work on early Carboniferous sites is shedding light on early vertebrate life on land. LeatherbackTurtle, Dermochelys coriacea 3 Leatherbacks are the largest living turtles. They have a wide geographical range, but their numbers are falling. Eggs are laid on tropical beaches, and hatchlings must fend for themselves against many perils. Only around one in a thousand leatherback hatchlings reach adulthood. With such a low survival rate, the harvesting of turtle eggs has had a devastating impact on leatherback populations. Nile Crocodile, Crocodylus niloticus 4 This skeleton was collected by Dr Hugh Cott (1900- 1987). -

Vocabulary: Sharks
Grades 11-12 - Vocabulary: Sharks Dermal Denticles – Tiny tooth-shaped scales that cover a shark’s body. Dermal Denticles have the same structure as teeth - enamel, dentine, pulp, epidermis, and dermis. Counter Shading - Having a dark dorsal or upper side and a lighter colored underside. Lateral Line – A row of sensors used by sharks and other fish, which detect vibrations. Cartilage – The material that makes up a shark’s skeleton (not bone), and is also found in our ears and nose. Basihyal - A sharks tongue, composed of a small piece of cartilage on the bottom of a sharks’ mouth. Carnivore - An animal that eats meat. Megalodon - An ancient shark that lived between 5 and 1.6 million years ago. Serrated Tooth - A tooth with a jagged edge that is used for sawing. Dorsal Fin - Primary fin located on the back of fishes and certain marine mammals. Pectoral Fins - Either of the anterior pairs of fins. Barbels - Sensory projections near the nostrils and mouth of some sharks, i.e. nurse sharks. They are whisker-like feelers used to taste and feel. Gills - Respiratory organs that fish use to absorb oxygen from the water in order to breathe. Snout - The tip of a shark’s head. Pup - A newly born or hatched shark. Claspers - Two finger like projections on the rear underside of male sharks. Ampullae of Lorenzini - Pores scattered about the head of sharks that are filled with a jellylike substance that can sense temperature change and weak electrical impulses given off by sick prey. Fusiform – A streamlined, oval shape body. -

Importance of Dewlap Display in Male Mating Success in Free- Ranging Brown Anoles (Anolis Sagrei) Author(S): Richard R
Importance of Dewlap Display in Male Mating Success in Free- Ranging Brown Anoles (Anolis sagrei) Author(s): Richard R. Tokarz, Ann V. Paterson, Stephen McMann Source: Journal of Herpetology, 39(1):174-177. 2005. Published By: The Society for the Study of Amphibians and Reptiles DOI: http://dx.doi.org/10.1670/0022-1511(2005)039[0174:IODDIM]2.0.CO;2 URL: http://www.bioone.org/doi/ full/10.1670/0022-1511%282005%29039%5B0174%3AIODDIM%5D2.0.CO %3B2 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. SHORTER COMMUNICATIONS Journal of Herpetology, Vol. 39, No. 1, pp. 174–177, 2005 Copyright 2005 Society for the Study of Amphibians and Reptiles Importance of Dewlap Display in Male Mating Success in Free-Ranging Brown Anoles (Anolis sagrei) 1,2 3,4 3,5 RICHARD R. -
Glossary of Common MCH Terms and Acronyms
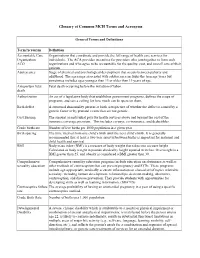
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

Onychophorology, the Study of Velvet Worms
Uniciencia Vol. 35(1), pp. 210-230, January-June, 2021 DOI: http://dx.doi.org/10.15359/ru.35-1.13 www.revistas.una.ac.cr/uniciencia E-ISSN: 2215-3470 [email protected] CC: BY-NC-ND Onychophorology, the study of velvet worms, historical trends, landmarks, and researchers from 1826 to 2020 (a literature review) Onicoforología, el estudio de los gusanos de terciopelo, tendencias históricas, hitos e investigadores de 1826 a 2020 (Revisión de la Literatura) Onicoforologia, o estudo dos vermes aveludados, tendências históricas, marcos e pesquisadores de 1826 a 2020 (Revisão da Literatura) Julián Monge-Nájera1 Received: Mar/25/2020 • Accepted: May/18/2020 • Published: Jan/31/2021 Abstract Velvet worms, also known as peripatus or onychophorans, are a phylum of evolutionary importance that has survived all mass extinctions since the Cambrian period. They capture prey with an adhesive net that is formed in a fraction of a second. The first naturalist to formally describe them was Lansdown Guilding (1797-1831), a British priest from the Caribbean island of Saint Vincent. His life is as little known as the history of the field he initiated, Onychophorology. This is the first general history of Onychophorology, which has been divided into half-century periods. The beginning, 1826-1879, was characterized by studies from former students of famous naturalists like Cuvier and von Baer. This generation included Milne-Edwards and Blanchard, and studies were done mostly in France, Britain, and Germany. In the 1880-1929 period, research was concentrated on anatomy, behavior, biogeography, and ecology; and it is in this period when Bouvier published his mammoth monograph. -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix. -

Post-Partum Hysterectomy (Removal of the Uterus/Womb After Giving Birth)
Post-partum hysterectomy (removal of the uterus/womb after giving birth) This leaflet explains what happens when a woman needs a post-partum hysterectomy following complications during giving birth. It explains why and how it is done, and what to expect afterwards. If there is anything you do not understand or if you have any questions, please speak to your midwife or doctor. What is post-partum hysterectomy? This is an operation that involves removal of the uterus (womb). This is an uncommon situation in the UK, with around 1 in 1000 women having this procedure done shortly after childbirth in this hospital, as there is a range of treatments used before such surgery which can save both future fertility and the mother’s life. It may be performed in an emergency to save the life of a woman with persistent bleeding after childbirth. Less frequently, it can be a planned procedure, often at the same time as Caesarean birth. Why is it performed? The most common reason is severe bleeding from the uterus that cannot be controlled by other measures. There is a link to Caesarean birth, particularly if the placenta for the most recent baby is both low in the uterus (placenta praevia), and deeply adherent (placenta grows too deeply into the uterine wall, known as placenta percreta or increta), so not separating fully after the birth of the baby. A more common cause of heavy bleeding is ‘uterine atony’, which is the inability of a womb to contract after the birth, as well as large or multiple fibroids and blood clotting problems. -

Mating Systems and Parental Investment Mating Systems
Mating systems and parental investment Mating systems Pattern of matings in a population green anole Antithesis = promiscuity Polygyny Polygyny Scramble: no attempts to defend females, resources horseshoe crabs Northern barred frog Female defense: must be clustered elk Montezuma’s oropendola Dulichiella spp. Polygyny Resource distribution Resource defense: males defend food, nest sites Distribution of females affects Red-winged blackbird Lamprologus cichlid males’ ability to guard them Males cannot monopolize wide-ranging females dunnock 1 Polygyny threshold Polygyny threshold Male with no other females (monogamy) Male with other female(s) polygyny threshold ??? Quality of male’s territory Polygyny threshold Male dominance polygyny When females and sage grouse Polygyny threshold = point at which it’s resources too dispersed, better to be polygynous on a good territory males compete Leks = communal display arenas hammerhead bat Uganda kob Leks Leks High variance in male mating success – 10-20% males achieve >50% copulations – one male got 75% copulations Classical lek: males display in sight of each other Exploded lek: males rely wire-tailed manakin on vocal communication, e.g. kakapo 2 Leks Leks • Hotshots • Hotshots – Females attracted to lek by dominant male – Females attracted to lek by dominant male • Hotspots – Leks located in high-use areas Leks Leks • Hotshots Position of most successful – Females attracted to lek by dominant male male territory shifts (hot shot?) • Hotspots black grouse – Leks located in high-use areas • Female -

Parent Information: Bleeding After Birth
What causes bleeding to increase? This information sheet aims to answer some commonly asked questions about bleeding after birth. An increase in bleeding can happen because: IMPORTANT: This is general information only. It is not intended as • Your uterus isn’t contracting properly advice for your individual circumstances. Ask your health care • There is tissue from the placenta in your uterus provider for more information. preventing it from contracting • You have an injury to your vagina, cervix or uterus Is vaginal bleeding normal after birth? causing bleeding Yes, vaginal bleeding (also called lochia) is completely • You have an infection normal after giving birth. Bleeding occurs if you have a vaginal birth or a caesarean section birth. Can you tell if your uterus is contracted normally? What is normal blood loss after birth? In the first 24 hours after birth, the top of your uterus In the first 1–2 days after your baby is born, bleeding is (the fundus) can be felt around the level of your belly usually bright red in colour. On the first day you may button. It will feel a bit like a grapefruit in size and soak up to one sanitary pad each hour. Over the next texture. As your uterus contracts, it will slowly decrease several days, the bleeding will slowly get less each day in size and be felt lower down on your abdomen. and change colour from bright red to a pink or brown Around 7–10 days after birth your uterus will have colour and then to a creamy colour. Most women will contracted so much that you can no longer feel it. -

Choices in Childbirth Pamphlet
Choices in childbirth @CedarsSinai #CedarsBabies © 2020 Cedars-Sinai 14434 (0220) My birth preferences Birth cannot be planned, but preferences can be shared. Birth preferences are the choices that are important to you. This document is a communication tool for you to share your preferences for labor, birth and recovery. Thank you for choosing Cedars-Sinai! We look forward to birthing with you. We believe that pregnancy and birth are natural experiences that are different for each woman and her My care team family. We honor all families and respect your birth choices. We will share information with My name: you, answer your questions and then make decisions together. When making decisions, it is Along with Cedars-Sinai nurses, residents and midwives, my care team includes: important to know what “evidence shows.” Evidence is the most up-to-date support from Support people: Obstetrician or Certified Nurse Midwife: research that helps parents and caregivers make informed choices. Pediatrician: Here are important things you should know: Labor preferences Birth preferences Newborn care I am planning a vaginal birth I would like the cord clamped: Skin-to-skin right after birth: I plan on laboring with: Right away Yes A “walking epidural” A few minutes after birth No Evidence shows that: Talk with your doctor to decide what is best for you. When mothers move and change positions, their labor A “standard labor epidural” Do you want to take your placenta home? Do you want the recommended tends to progress better. That means you should walk, change IV pain medication Yes. I will arrange for it to be picked up/ newborn medications? When there are no problems in pregnancy or during positions and try many of our support tools (birthing ball, squat Unmedicated comfort techniques: removed from the room in the first hour Vitamin K bar, rocking chair).