Cjfr-2020-0094.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Rubus Arcticus Ssp. Acaulis Is Also Appreciated
Rubus arcticus L. ssp. acaulis (Michaux) Focke (dwarf raspberry): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project October 18, 2006 Juanita A. R. Ladyman, Ph.D. JnJ Associates LLC 6760 S. Kit Carson Cir E. Centennial, CO 80122 Peer Review Administered by Society for Conservation Biology Ladyman, J.A.R. (2006, October 18). Rubus arcticus L. ssp. acaulis (Michaux) Focke (dwarf raspberry): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http:// www.fs.fed.us/r2/projects/scp/assessments/rubusarcticussspacaulis.pdf [date of access]. ACKNOWLEDGMENTS The time spent and help given by all the people and institutions mentioned in the reference section are gratefully acknowledged. I would also like to thank the Wyoming Natural Diversity Database, in particular Bonnie Heidel, and the Colorado Natural Heritage Program, in particular David Anderson, for their generosity in making their records available. The data provided by Lynn Black of the DAO Herbarium and National Vascular Plant Identification Service in Ontario, Marta Donovan and Jenifer Penny of the British Columbia Conservation Data Center, Jane Bowles of University of Western Ontario Herbarium, Dr. Kadri Karp of the Aianduse Instituut in Tartu, Greg Karow of the Bighorn National Forest, Cathy Seibert of the University of Montana Herbarium, Dr. Anita Cholewa of the University of Minnesota Herbarium, Dr. Debra Trock of the Michigan State University Herbarium, John Rintoul of the Alberta Natural Heritage Information Centre, and Prof. Ron Hartman and Joy Handley of the Rocky Mountain Herbarium at Laramie, were all very valuable in producing this assessment. -

Natural Resource Inventory Smith-Sargent
NATURAL RESOURCE INVENTORY of the SMITH-SARGENT ROAD PROPERTY Holderness, NH FINAL REPORT [Smith-Sargent Property Upper Marsh as seen from south boundary] Compiled by: Dr. Rick Van de Poll Ecosystem Management Consultants 30 N. Sandwich Rd. Center Sandwich, NH 03227 603-284-6851 [email protected] Submitted to: Holderness Conservation Commission June 30, 2016 i SUMMARY Between October 2015 and June 2016 a comprehensive natural resources inventory (NRI) was completed by Ecosystem Management Consultants (EMC) of Sandwich, NH on the 8.5-acre town conservation land at the corner of Sargent Road and Smith Road in Holderness, NH. Managed by the Holderness Conservation Commission (HCC), this parcel was obtained largely for the complex wetland system that occupies more than 65% of the parcel. The purpose of the NRI was to inform the town about the qualities of the natural resources on the lot, as well as to determine whether or not the site would be suitable for limited environmental education for the general public. Three site visits were conducted at the Sargent-Smith Road Property for the purpose of gathering NRI data. A fourth visit was also made on November 15, 2015 for the purpose of educating the HCC and other town officials about the extent and functional value of the wetlands on the parcel. The first field visit in October provided an initial review of the location of the parcel, the boundary of the wetland, and the plant and animal resources present. A second site visit in January was held for the purpose of tracking mammals during good snow cover. -

Differential Colonization by Ecto-, Arbuscular and Ericoid Mycorrhizal Fungi in Forested Wetland Plants. by Amanda Marie Griffin
Differential colonization by ecto-, arbuscular and ericoid mycorrhizal fungi in forested wetland plants. By Amanda Marie Griffin A Thesis Submitted to Saint Mary’s University, Halifax, Nova Scotia in Partial Fulfillment of the Requirements for the Degree of Master of Science in Applied Science. August, 2019, Halifax, Nova Scotia © Amanda Marie Griffin, 2019 _____________________________ Approved: Gavin Kernaghan Supervisor _____________________ Approved: Dr. Jeremy Lundholm Supervisory Committee Member _____________________ Approved: Dr. Kevin Keys Supervisory Committee Member ______________________ Approved: Dr. Pedro Antunes External Examiner Date: August 26th, 2019 Differential colonization by ecto-, arbuscular and ericoid mycorrhizal fungi in forested wetland plants. by Amanda Marie Griffin Abstract The roots of most land plants are colonized by mycorrhizal fungi under normal soil conditions, yet the influence of soil moisture on different types of mycorrhizal symbioses is poorly understood. In wet soils, colonization of woody plants by ectomycorrhizal (ECM) fungi tends to be poor, and colonization of herbaceous plants by arbuscular mycorrhizal (AM) is highly variable. However, little information is available on the influence of soil moisture on the colonization of ericaceous roots by ericoid mycorrhizal (ErM) fungi. Colonization was assessed microscopically in the ECM plant Pinus strobus, two AM plants (Cornus canadensis and Lysimachia borealis) and two ErM plants (Kalmia angustifolia and Gaultheria hispidula) along two upland to wetland gradients in Southwestern Nova Scotia. For the ErM plants, fungal ITS sequencing was used to assess community structure. The data indicate that ErM colonization increases with soil moisture in forested wetlands and is associated with distinctive fungal communities. August 26th, 2019 2 For Heidi, my constant companion. -

Trees, Shrubs and Vines of Toronto Is Not a Field Guide in the Typical Sense
WINNER OALA AWARD FOR SERVICE TO THE ENVIRONMENT TREES, SHRUBS & VINES OF TORONTO A GUIDE TO THEIR REMARKABLE WORLD City of Toronto Biodiversity Series Imagine a Toronto with flourishing natural habitats and an urban environment made safe for a great diversity of wildlife. Envision a city whose residents treasure their daily encounters with the remarkable and inspiring world of nature, and the variety of plants and animals who share this world. Take pride in a Toronto that aspires to be a world leader in the development of urban initiatives that will be critical to the preservation of our flora and fauna. PO Cover photo: “Impact,” sugar maple on Taylor Creek Trail by Yasmeen (Sew Ming) Tian photo: Jenny Bull Ohio buckeye, Aesculus glabra: in full flower on Toronto Island (above); the progression of Ohio buckeye flowers (counterclockwise on next page) from bud, to bud burst, to flower clusters elongating as leaves unfurl, to an open flower cluster City of Toronto © 2015 City of Toronto © 2016 ISBN 978-1-895739-77-0 “Animals rule space, Trees rule time.” – Francis Hallé 11 “Indeed, in its need for variety and acceptance of randomness, a flourishing TABLE OF CONTENTS natural ecosystem is more like a city than like a plantation. Perhaps it will be the city that reawakens our understanding and appreciation of nature, in all its teeming, unpredictable complexity.” – Jane Jacobs Welcome from Margaret Atwood and Graeme Gibson ............ 2 For the Love of Trees................................. 3 The Story of the Great Tree of Peace ...................... 4 What is a Tree?..................................... 6 Classifying Trees .................................... 9 Looking at Trees: Conifers ........................... -

Vegetation Classification and Mapping Project Report
U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Project Report Revised Edition – October 2003 Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U. S. Department of the Interior, U. S. Geological Survey. USGS-NPS Vegetation Mapping Program Acadia National Park U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Sara Lubinski and Kevin Hop U.S. Geological Survey Upper Midwest Environmental Sciences Center and Susan Gawler Maine Natural Areas Program This report produced by U.S. Department of the Interior U.S. Geological Survey Upper Midwest Environmental Sciences Center 2630 Fanta Reed Road La Crosse, Wisconsin 54603 and Maine Natural Areas Program Department of Conservation 159 Hospital Street 93 State House Station Augusta, Maine 04333-0093 In conjunction with Mike Story (NPS Vegetation Mapping Coordinator) NPS, Natural Resources Information Division, Inventory and Monitoring Program Karl Brown (USGS Vegetation Mapping Coordinator) USGS, Center for Biological Informatics and Revised Edition - October 2003 USGS-NPS Vegetation Mapping Program Acadia National Park Contacts U.S. Department of Interior United States Geological Survey - Biological Resources Division Website: http://www.usgs.gov U.S. Geological Survey Center for Biological Informatics P.O. Box 25046 Building 810, Room 8000, MS-302 Denver Federal Center Denver, Colorado 80225-0046 Website: http://biology.usgs.gov/cbi Karl Brown USGS Program Coordinator - USGS-NPS Vegetation Mapping Program Phone: (303) 202-4240 E-mail: [email protected] Susan Stitt USGS Remote Sensing and Geospatial Technologies Specialist USGS-NPS Vegetation Mapping Program Phone: (303) 202-4234 E-mail: [email protected] Kevin Hop Principal Investigator U.S. -

Summer 2013 Volume 24 Issue 2
Quarterly Newsletter Summer 2013 Volume 24 Issue 2 Articles Inside: A Sticky Situation: Salvia glutinosa in A Sticky Situation 1 Southeastern New York Plant Conservationist 2 by Nava Tabak Solanum dulcamara 3 Notes 6 In the young forests of southeastern plant was Salvia glutinosa. Annual Meeting Recap 7 Common Mosses Review 9 New York, invasive plants are Salvia glutinosa is a perennial herb A New Botanical Duo 10 ubiquitous and plentiful, and it is easy native to Europe and western Asia, Bark Review 11 to be lulled into the sense that we where it grows in wooded Field Trip Report 12 know all our invasive plants. After all, mountainous areas. The plant grows with garlic mustard, Japanese 50–100 cm tall, has opposite, toothed barberry, Asiatic bittersweet, tree-of- leaves with hastate bases, yellow heaven, and many others, what more corollas with brown markings, and could possibly compete here? And sticky glandular hairs on the leaves, with relatively high population stem, and calices (Tutin et al. 1972). densities, what are the chances of a As with many other species in the truly invasive species going genus, S. glutinosa is used in undetected? Imagine my surprise then, gardening, and is known to when in the fall of 2009 I encountered horticulturalists as Jupiter’s sage or a plant I did not recognize growing sticky sage. abundantly in the forest understory on With its identity revealed, I began lands near the Appalachian Trail in the to search more broadly for records of town of Dover (southeastern Dutchess this species’ introduction and County). -

Species Profile: Minnesota DNR
Species profile: Minnesota DNR events | a-z list | newsroom | about DNR | contact us Recreation | Destinations | Nature | Education / safety | Licenses / permits / regs. Home > Nature > Rare Species Guide > Keyword Search | A-Z Search | Filtered Search Botrychium campestre W.H. Wagner & Farrar ex W.H. & F. Prairie Wagner Moonwort MN Status: Basis for Listing special concern Federal Status: Botrychium campestre was first none discovered in 1982 and described eight CITES: years later (Wagner and Wagner 1990). none Until that time, no one knew that USFS: Botrychium spp. (moonworts) occurred in none prairies. The discovery sparked considerable interest among botanists in Group: finding more sites of this species, and in vascular plant trying to find out if other undescribed Class: moonworts could be found. Results have Ophioglossopsida been quite impressive; we now know that Order: B. campestre ranges across the whole Ophioglossales continent. Botanists have also discovered Family: previously undescribed species of Ophioglossaceae Botrychium from prairies and a variety of Life Form: prairie-like habitats. The actual rarity of forb B. campestre is difficult to judge at this Longevity: time. There are now numerous records, perennial but they are the result of an Leaf Duration: unprecedented search effort. Further deciduous searches will undoubtedly discover Water Regime: additional sites, and it is possible that at terrestrial some time in the future B. campestre will Soils: be thought of as relatively common. Map Interpretation sand, loam Botrychium campestre was listed as a Light: special concern species in Minnesota in full sun 1996. Habitats: Upland Prairie Description Botrychium campestre is a small, Best time to see: inconspicuous fern that can be very difficult to find. -

100 Years of Change in the Flora of the Carolinas
ASTERACEAE 224 Zinnia Linnaeus 1759 (Zinnia) A genus of about 17 species, herbs, of sw. North America south to South America. References: Smith in FNA (2006c); Cronquist (1980)=SE. 1 Achenes wingless; receptacular bracts (chaff) toothed or erose on the lip..............................................................Z. peruviana 1 Achenes winged; receptacular bracts (chaff) with a differentiated fimbriate lip........................................................Z. violacea * Zinnia peruviana (Linnaeus) Linnaeus, Zinnia. Cp (GA, NC, SC): disturbed areas; rare (commonly cultivated), introduced from the New World tropics. May-November. [= FNA, K, SE; ? Z. pauciflora Linnaeus – S] * Zinnia violacea Cavanilles, Garden Zinnia. Cp (GA, NC, SC): disturbed areas; rare (commonly cultivated), introduced from the New World tropics. May-November. [= FNA, K; ? Z. elegans Jacquin – S, SE] BALSAMINACEAE A. Richard 1822 (Touch-me-not Family) A family of 2 genera and 850-1000 species, primarily of the Old World tropics. References: Fischer in Kubitzki (2004). Impatiens Linnaeus (Jewelweed, Touch-me-not, Snapweed, Balsam) A genus of 850-1000 species, herbs and subshrubs, primarily tropical and north temperate Old World. References: Fischer in Kubitzki (2004). 1 Corolla purple, pink, or white; plants 3-6 (-8) dm tall; stems puberulent or glabrous; [cultivated alien, rarely escaped]. 2 Sepal spur strongly recurved; stems puberulent..............................................................................................I. balsamina 2 Sepal spur slightly -
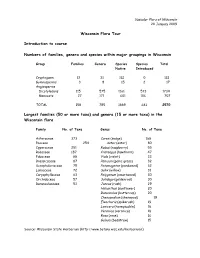
Wisconsin Flora Tour Introduction to Course Numbers of Families, Genera
Vascular Flora of Wisconsin 20 January 2009 Wisconsin Flora Tour Introduction to course Numbers of families, genera and species within major groupings in Wisconsin Group Families Genera Species Species Total Native Introduced Cryptogams 13 31 112 0 112 Gymnosperms 3 8 15 2 17 Angiosperms Dicotyledons 115 575 1161 573 1734 Monocots 27 171 601 106 707 TOTAL 158 785 1889 681 2570 Largest families (50 or more taxa) and genera (15 or more taxa) in the Wisconsin flora Family No. of Taxa Genus No. of Taxa Asteraceae 373 Carex (sedge) 168 Poaceae 254 Aster (aster) 80 Cyperaceae 251 Rubus (raspberry) 55 Rosaceae 187 Crateagus (hawthorn) 47 Fabaceae 88 Viola (violet) 33 Brassicaceae 87 Panicum (panic grass) 32 Scrophulariaceae 75 Potamogeton (pondweed) 32 Lamiaceae 72 Salix (willow) 31 Caryophyllaceae 63 Polygonum (smartweed) 30 Orchidaceae 57 Solidago (goldenrod) 30 Ranunculaceaee 53 Juncus (rush) 29 Helianthus (sunflower) 20 Ranunculus (buttercup) 20 Chenopodium (chenopod) 19 Eleocharis (spikerush) 19 Lonicera (honeysuckle) 18 Veronica (veronica) 18 Rosa (rose) 16 Galium (bedstraw) 15 Source: Wisconsin State Herbarium (http://www.botany.wisc.edu/herbarium/) Four major floristic elements in the Wisconsin flora Boreal Alleghenian Ozarkian Prairie Two floristic provinces Northern hardwood Prairie forests Tension Zone Brief look at four plant communities Beech maple or southern mesic Oak forest or southern xeric Prairie Bog or fen Vascular Flora of Wisconsin 22 January 2009 Nomenclature and Vascular Cryptogams I Nomenclature vs. Classification Rank -

Njplantlist.Pdf
List of Endangered Plant Species and Plant Species of Concern June 2016 Scientific Name Common Name G Rank S Rank Federal Status State Status Other Status Abies balsamea Balsam Fir G5 S1 E LP, HL Acorus americanus American Sweetflag G5 S1? HL Actaea rubra var. rubra Red Baneberry G5T5 S2 HL Adlumia fungosa Climbing Fumitory G4 S2 HL Aeschynomene virginica Sensitive Joint-vetch G2 S1 LT E LP, HL Agalinis auriculata Ear-leaf False Foxglove G3 SX HL Agalinis fasciculata Pine Barren Foxglove G5 S3 HL Agalinis paupercula var. paupercula Small-flower False Foxglove G5T5 S2 HL Agastache nepetoides Yellow Giant-hyssop G5 S2 HL Agastache scrophulariifolia Purple Giant-hyssop G4 S2 HL Agrimonia microcarpa Small-fruit Grooveburr G5 S2 HL Agrostis geminata Ticklegrass G5 S1? HL Alisma triviale Large Water-plantain G5 S1 E LP, HL Alopecurus aequalis var. aequalis Short-awn Meadow-foxtail G5T5 S2 HL Alopecurus carolinianus Tufted Meadow-foxtail G5 S3 HL Amaranthus pumilus Seabeach Amaranth G2 S1 LT E LP, HL Amelanchier humilis Low Service-berry G5 S1S2 HL Amelanchier nantucketensis Nantucket Service-berry G3Q S1 HL Amelanchier sanguinea var. sanguinea Round-leaf Service-berry G5T5 S1.1 E LP, HL Amelanchier stolonifera Running Service-berry G5 S3 HL Amianthium muscitoxicum Fly Poison G4G5 S2 HL Ammannia latifolia Koehn's Toothcup G5 S1 E LP, HL Andromeda polifolia var. glaucophylla Bog Rosemary G5T5 S1 E LP, HL Andropogon glomeratus var. hirsutior Hairy Beardgrass G5T5 SH.1 HL Andropogon gyrans Elliott's Beardgrass G5 S2 HL Andropogon ternarius var. ternarius Silvery Beardgrass G5T5? S2 HL Anemone canadensis Canada Anemone G5 SX HL Anemone cylindrica Long-head Anemone G5 S1 E LP, HL Anemone virginiana var. -

Recommended Planting Species County of Brant
PLANNING DIVISION COUNTY OF BRANT RECOMMENDED PLANTING SPECIES COUNTY OF BRANT August 2005 January 16, 2008 PURPOSE These Guidelines were created to assist with the selection of plant materials that are to be used and not used when preparing landscaping plans within the County. The intent is to protect natural areas from invasive species that can have the affect of destroying natural habitat. By applying these standards to development applications, the County will assist in limiting the potential negative impacts invasive species can create, and at the same time, provide species that are native to the County. The materials to be used should be tolerant to urban conditions, and are encouraged to be of native species. The Guidelines have been created with the assistance of the Grand River Conservation Authority, Jeff Thompson of Thompson Environmental Planning and Design, and County Staff. Species Recommended For Use NATIVE TREE SPECIES BOTANICAL NAME COMMON NAME Pinaceae Pine Family Abies balsamea Balsam fir Larix laricina Tamarack Picea mariana Black spruce Pinus strobus Eastern white pine Tsuga canadensis Eastern hemlock Cupressaceae Cypress Family Juniperus virginiana Eastern red cedar Thuja occidentalis Eastern white cedar Salicaceae Willow Family Populus balsamifera Balsam poplar Populus deltoides Eastern cottonwood Populus grandidentata Large tooth aspen Populus tremuloides Trembling aspen Salix amygdaloides Peach-leaf willow Salix bebbiana Bebb or Beaked willow Salix lucida Shining willow Salix discolor Pussy willow Salix nigra