P2 Receptors in Cardiovascular Regulation and Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
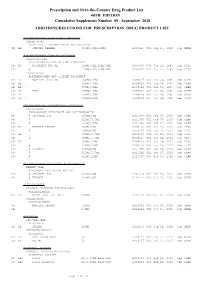
Additions and Deletions to the Drug Product List
Prescription and Over-the-Counter Drug Product List 40TH EDITION Cumulative Supplement Number 09 : September 2020 ADDITIONS/DELETIONS FOR PRESCRIPTION DRUG PRODUCT LIST ACETAMINOPHEN; BUTALBITAL; CAFFEINE TABLET;ORAL BUTALBITAL, ACETAMINOPHEN AND CAFFEINE >A> AA STRIDES PHARMA 325MG;50MG;40MG A 203647 001 Sep 21, 2020 Sep NEWA ACETAMINOPHEN; CODEINE PHOSPHATE SOLUTION;ORAL ACETAMINOPHEN AND CODEINE PHOSPHATE >D> AA WOCKHARDT BIO AG 120MG/5ML;12MG/5ML A 087006 001 Jul 22, 1981 Sep DISC >A> @ 120MG/5ML;12MG/5ML A 087006 001 Jul 22, 1981 Sep DISC TABLET;ORAL ACETAMINOPHEN AND CODEINE PHOSPHATE >A> AA NOSTRUM LABS INC 300MG;15MG A 088627 001 Mar 06, 1985 Sep CAHN >A> AA 300MG;30MG A 088628 001 Mar 06, 1985 Sep CAHN >A> AA ! 300MG;60MG A 088629 001 Mar 06, 1985 Sep CAHN >D> AA TEVA 300MG;15MG A 088627 001 Mar 06, 1985 Sep CAHN >D> AA 300MG;30MG A 088628 001 Mar 06, 1985 Sep CAHN >D> AA ! 300MG;60MG A 088629 001 Mar 06, 1985 Sep CAHN ACETAMINOPHEN; HYDROCODONE BITARTRATE TABLET;ORAL HYDROCODONE BITARTRATE AND ACETAMINOPHEN >A> @ CEROVENE INC 325MG;5MG A 211690 001 Feb 07, 2020 Sep CAHN >A> @ 325MG;7.5MG A 211690 002 Feb 07, 2020 Sep CAHN >A> @ 325MG;10MG A 211690 003 Feb 07, 2020 Sep CAHN >D> AA VINTAGE PHARMS 300MG;5MG A 090415 001 Jan 24, 2011 Sep DISC >A> @ 300MG;5MG A 090415 001 Jan 24, 2011 Sep DISC >D> AA 300MG;7.5MG A 090415 002 Jan 24, 2011 Sep DISC >A> @ 300MG;7.5MG A 090415 002 Jan 24, 2011 Sep DISC >D> AA 300MG;10MG A 090415 003 Jan 24, 2011 Sep DISC >A> @ 300MG;10MG A 090415 003 Jan 24, 2011 Sep DISC >D> @ XIROMED 325MG;5MG A 211690 -

Cangrelor Ameliorates CLP-Induced Pulmonary Injury in Sepsis By
Luo et al. Eur J Med Res (2021) 26:70 https://doi.org/10.1186/s40001-021-00536-4 European Journal of Medical Research RESEARCH Open Access Cangrelor ameliorates CLP-induced pulmonary injury in sepsis by inhibiting GPR17 Qiancheng Luo1†, Rui Liu2†, Kaili Qu3, Guorong Liu1, Min Hang1, Guo Chen1, Lei Xu1, Qinqin Jin1 , Dongfeng Guo1* and Qi Kang1* Abstract Background: Sepsis is a common complication of severe wound injury and infection, with a very high mortality rate. The P2Y12 receptor inhibitor, cangrelor, is an antagonist anti-platelet drug. Methods: In our study, we investigated the protective mechanisms of cangrelor in CLP-induced pulmonary injury in sepsis, using C57BL/6 mouse models. Results: TdT-mediated dUTP Nick-End Labeling (TUNEL) and Masson staining showed that apoptosis and fbrosis in lungs were alleviated by cangrelor treatment. Cangrelor signifcantly promoted surface expression of CD40L on platelets and inhibited CLP-induced neutrophils in Bronchoalveolar lavage fuid (BALF) (p < 0.001). We also found that cangrelor decreased the infammatory response in the CLP mouse model and inhibited the expression of infamma- tory cytokines, IL-1β (p < 0.01), IL-6 (p < 0.05), and TNF-α (p < 0.001). Western blotting and RT-PCR showed that cangre- lor inhibited the increased levels of G-protein-coupled receptor 17 (GPR17) induced by CLP (p < 0.001). Conclusion: Our study indicated that cangrelor repressed the levels of GPR17, followed by a decrease in the infam- matory response and a rise of neutrophils in BALF, potentially reversing CLP-mediated pulmonary injury during sepsis. Keywords: Sepsis, Infammation, Cangrelor, Platelet, GPR17 Background Te lung is one of the initial target organ of the systemic Sepsis is a serious disease and will lead a high mortal- infammatory response caused by sepsis, leading to alve- ity rate of approximately 22% in all over the world [1]. -

Kengrexal, INN-Cangrelor Tetrasodium
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Kengrexal 50 mg powder for concentrate for solution for injection/infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains cangrelor tetrasodium corresponding to 50 mg cangrelor. After reconstitution 1 mL of concentrate contains 10 mg cangrelor. After dilution 1 mL of solution contains 200 micrograms cangrelor. Excipient with known effect Each vial contains 52.2 mg sorbitol. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for injection/infusion. White to off-white lyophilised powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable. 4.2 Posology and method of administration Kengrexal should be administered by a physician experienced in either acute coronary care or in coronary intervention procedures and is intended for specialised use in an acute and hospital setting. Posology The recommended dose of Kengrexal for patients undergoing PCI is a 30 micrograms/kg intravenous bolus followed immediately by 4 micrograms/kg/min intravenous infusion. The bolus and infusion should be initiated prior to the procedure and continued for at least two hours or for the duration of the procedure, whichever is longer. At the discretion of the physician, the infusion may be continued for a total duration of four hours, see section 5.1. -

Prasugrel Mylan 10 Mg Film-Coated Tablets Prasugrel
Package leaflet: Information for the user Prasugrel Mylan 5 mg film-coated tablets Prasugrel Mylan 10 mg film-coated tablets prasugrel Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. – Keep this leaflet. You may need to read it again. – If you have any further questions, ask your doctor or pharmacist. – This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. – If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Prasugrel Mylan is and what it is used for 2. What you need to know before you take Prasugrel Mylan 3. How to take Prasugrel Mylan 4. Possible side effects 5. How to store Prasugrel Mylan 6. Contents of the pack and other information 1. What Prasugrel Mylan is and what it is used for Prasugrel Mylan, which contains the active substance prasugrel, belongs to a group of medicines called antiplatelet agents. Platelets are very small cell particles that circulate in the blood. When a blood vessel is damaged, for example if it is cut, platelets clump together to help form a blood clot (thrombus). Therefore, platelets are essential to help stop bleeding. If clots form within a hardened blood vessel such as an artery they can be very dangerous as they can cut off the blood supply, causing a heart attack (myocardial infarction), stroke or death. -

Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations
molecules Review Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations Deepak Gupta 1, Deepak Bhatia 2 ID , Vivek Dave 3 ID , Vijaykumar Sutariya 4 and Sheeba Varghese Gupta 4,* 1 Department of Pharmaceutical Sciences, School of Pharmacy, Lake Erie College of Osteopathic Medicine, Bradenton, FL 34211, USA; [email protected] 2 ICPH Fairfax Bernard J. Dunn School of Pharmacy, Shenandoah University, Fairfax, VA 22031, USA; [email protected] 3 Wegmans School of Pharmacy, St. John Fisher College, Rochester, NY 14618, USA; [email protected] 4 Department of Pharmaceutical Sciences, USF College of Pharmacy, Tampa, FL 33612, USA; [email protected] * Correspondence: [email protected]; Tel.: +01-813-974-2635 Academic Editor: Peter Wipf Received: 7 June 2018; Accepted: 13 July 2018; Published: 14 July 2018 Abstract: The physicochemical and biological properties of active pharmaceutical ingredients (APIs) are greatly affected by their salt forms. The choice of a particular salt formulation is based on numerous factors such as API chemistry, intended dosage form, pharmacokinetics, and pharmacodynamics. The appropriate salt can improve the overall therapeutic and pharmaceutical effects of an API. However, the incorrect salt form can have the opposite effect, and can be quite detrimental for overall drug development. This review summarizes several criteria for choosing the appropriate salt forms, along with the effects of salt forms on the pharmaceutical properties of APIs. In addition to a comprehensive review of the selection criteria, this review also gives a brief historic perspective of the salt selection processes. Keywords: chemistry; salt; water solubility; routes of administration; physicochemical; stability; degradation 1. -

A Comparative Study of Molecular Structure, Pka, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs
International Journal of Molecular Sciences Article A Comparative Study of Molecular Structure, pKa, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs Milan Remko 1,*, Anna Remková 2 and Ria Broer 3 1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University in Bratislava, Odbojarov 10, SK-832 32 Bratislava, Slovakia 2 Department of Internal Medicine, Faculty of Medicine, Slovak Medical University, Limbová 12, SK–833 03 Bratislava, Slovakia; [email protected] 3 Department of Theoretical Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +421-2-5011-7291 Academic Editor: Michael Henein Received: 18 February 2016; Accepted: 11 March 2016; Published: 19 March 2016 Abstract: Theoretical chemistry methods have been used to study the molecular properties of antiplatelet agents (ticlopidine, clopidogrel, prasugrel, elinogrel, ticagrelor and cangrelor) and several thiol-containing active metabolites. The geometries and energies of most stable conformers of these drugs have been computed at the Becke3LYP/6-311++G(d,p) level of density functional theory. Computed dissociation constants show that the active metabolites of prodrugs (ticlopidine, clopidogrel and prasugrel) and drugs elinogrel and cangrelor are completely ionized at pH 7.4. Both ticagrelor and its active metabolite are present at pH = 7.4 in neutral undissociated form. The thienopyridine prodrugs ticlopidine, clopidogrel and prasugrel are lipophilic and insoluble in water. Their lipophilicity is very high (about 2.5–3.5 logP values). The polar surface area, with regard to the structurally-heterogeneous character of these antiplatelet drugs, is from very large interval of values of 3–255 Å2. -

Expression of Dual Nucleotidescysteinylleukotrienes
J. Cell. Mol. Med. Vol 18, No 9, 2014 pp. 1785-1796 Expression of dual Nucleotides/Cysteinyl-Leukotrienes Receptor GPR17 in early trafficking of cardiac stromal cells after myocardial infarction Simona Cosentino a, #, Laura Castiglioni b, #, Francesca Colazzo a, #, Elena Nobili a, Elena Tremoli b, Patrizia Rosa c, Maria P. Abbracchio b, #, Luigi Sironi b, #, Maurizio Pesce d, #, * a Laboratorio di Biologia e Biochimica dell’Aterotrombosi, Centro Cardiologico Monzino, IRCCS, Milan, Italy b Dipartimento di Scienze Farmacologiche e Biomolecolari, Universita di Milano, Milan, Italy c Dipartimento di Biotecnologie Mediche e Medicina Traslazionale (BIOMETRA), Istituto di Neuroscienze, Milan, Italy d Laboratorio di Ingegneria Tissutale Cardiovascolare, Centro Cardiologico Monzino, IRCCS, Milan, Italy Received: November 8, 2013; Accepted: March 25, 2014 Abstract GPR17 is a Gi-coupled dual receptor activated by uracil-nucleotides and cysteinyl-leukotrienes. These mediators are massively released into hypoxic tissues. In the normal heart, GPR17 expression has been reported. By contrast, its role in myocardial ischaemia has not yet been assessed. In the present report, the expression of GPR17 was investigated in mice before and at early stages after myocardial infarction by using immunofluorescence, flow cytometry and RT-PCR. Before induction of ischaemia, results indicated the presence of the receptor in a pop- ulation of stromal cells expressing the stem-cell antigen-1 (Sca-1). At early stages after ligation of the coronary artery, the receptor was expressed in Sca-1+ cells, and cells stained with Isolectin-B4 and anti-CD45 antibody. GPR17+ cells also expressed mesenchymal marker CD44. GPR17 function was investigated in vitro in a Sca-1+/CD31À cell line derived from normal hearts. -

Dissertation.Pdf
UNIVERSITÄTSKLINIKUM HAMBURG-EPPENDORF Zentrum für Experimentelle Medizin, Institut für Experimentelle Pharmakologie und Toxikologie Direktor: Professor T. Eschenhagen Thrombus-Targeted Theranostic Microbubbles for Simultaneous Ultrasound Diagnosis and Therapy of Thrombosis. Title page Dissertation zur Erlangung des Grades eines Doktors der Medizin an der Medizinischen Fakultät der Universität Hamburg. vorgelegt von: Yannik Andreas Gkanatsas aus Bremen Hamburg 2017 Angenommen von der Medizinischen Fakultät der Universität Hamburg am: 13.12.2017 Veröffentlicht mit Genehmigung der Medizinischen Fakultät der Universität Hamburg. Prüfungsausschuss, der Vorsitzende: Prof. Dr. Thomas Eschenhagen Prüfungsausschuss, zweite Gutachterin: Prof. Dr. Renate Bonin-Schnabel Prüfungsausschuss, dritter Gutachter: PD Dr. Florian Langer 2 Table of contents Title page ..................................................................................................................... 1 Table of contents ......................................................................................................... 3 List of Figures: ............................................................................................................. 6 List of Tables: .............................................................................................................. 7 Chapter 1. Introduction ............................................................................................. 8 1.1 Cardiovascular Disease (CVD) ......................................................................................... -

Salts of Therapeutic Agents: Chemical, Physicochemical and Biological
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 16 April 2018 doi:10.20944/preprints201804.0187.v1 1 Review 2 Salts of Therapeutic Agents: Chemical, 3 Physicochemical and Biological Considerations 4 Deepak Gupta 1, Deepak Bhatia 2, Vivek Dave3, Vijaykumar Sutariya 4 and Sheeba Varghese 5 Gupta4* 6 1 Lake Eerie College of Osteopathic Medicine , School of Pharmacy, Pharmaceutical Sciences , Bradenton , FL , USA; 7 email: [email protected] 8 2 Shenandoah University - ICPH Fairfax Bernard J. Dunn School of Pharmacy, Fairfax, VA 22031USA; email: 9 [email protected] 10 3 Wegmans School of Pharmacy, St. John Fisher College, Rochester, NY, USA; email: [email protected] 11 4 Department of Pharmaceutical Sciences, USF College of Pharmacy, Tampa, FL, USA; email: 12 [email protected] 13 * Correspondence: [email protected]; Tel.: +01-813-974-2635 14 15 Abstract: Choice of the salts of therapeutic agents or active pharmaceutical ingredients (API) is 16 based on the physicochemical properties of API and the dosage form considerations. The 17 appropriate salt can have positive effect on overall therapeutic and pharmaceutical effects of API. 18 However, the incorrect salt form can negatively affect the overall pharmaceutical outcomes of the 19 API. This review addresses various criteria for choosing appropriate salt form along with the effect 20 of salt forms on API’s pharmaceutical properties. In addition to comprehensive review of the 21 criteria, this review also gives a brief historic perspective of the salt selection process. 22 23 Keywords: Chemistry, salt, water solubility, routes of administration, physicochemical, stability, 24 degradation 25 1. Introduction 26 Salt of an Active Pharmaceutical Ingredient (API) often formed to achieve desirable formulation 27 properties. -

New Drugs: T-Score for Transparency
New drugs: T-score for transparency Access to information about drugs is essential for the quality For 2011, the T-score has been revised to include the AusPAR. use of medicines. Pharmaceutical companies and regulatory agencies, such as the Therapeutic Goods Administration The revised T-scores will be as follows: (TGA), hold large quantities of information about individual drugs, but do not always share this information. To encourage T T T manufacturer provided complete clinical evaluation transparency, Australian Prescriber rates companies' willingness to provide clinical information about new drugs. T T manufacturer provided additional useful Table 1 shows how the companies have performed between information January 2009 and December 2010. The TGA is now publishing Australian Public Assessment T manufacturer provided the AusPAR and/or the Reports (AusPARs) for prescription medicines. While the product information Editorial Executive Committee welcomes this move to greater transparency, it will still ask companies to provide the clinical T manufacturer declined to supply data evaluations for their new products. While there are similarities, the AusPAR may not include all the details found in the X manufacturer did not respond to request for data regulator's clinical evaluation. Table 1 Pharmaceutical company responses to requests for clinical evaluation data for drugs marketed Jan 2009 – Dec 2010 Company Drug T T T manufacturer provided clinical evaluation Amgen denosumab Ferring degarelix Pfizer anidulafungin eletriptan PharmaLink cilostazol Phebra caffeine citrate Roche methoxy polyethylene glycol-epoetin beta tocilizumab Sanofi Pasteur H5N1 influenza vaccine Shire icatibant Wyeth methylnaltrexone T T manufacturer provided additional useful information Abbott omega-3-acid ethyl esters Celgene azacitidine Commercial Eyes melatonin CSL H1N1 influenza vaccine Japanese encephalitis vaccine Eli Lilly prasugrel Table continued.. -

Cardioprotection by Very Mild Hypothermia in Mice
67 Brief Report Cardioprotection by very mild hypothermia in mice Betül Knoop1,2#, David Naguib1,2#, Lisa Dannenberg1,2, Carolin Helten1,2, Saif Zako1,2, Christian Jung1,2, Bodo Levkau3, Maria Grandoch4, Malte Kelm1,2, Tobias Zeus1,2, Amin Polzin1,2 1Department of Cardiology, Pulmonology, and Vascular Medicine, Medical Faculty of the Heinrich Heine University Düsseldorf, Düsseldorf, Germany; 2Cardiovascular Research Institute Düsseldorf (CARID), Düsseldorf, Germany; 3Institute of Pathophysiology, West German Heart and Vascular Center, University Hospital Essen, University of Duisburg-Essen, Essen, Germany; 4Institute for Pharmacology and Clinical Pharmacology, Heinrich Heine University Düsseldorf, Düsseldorf, Germany #These authors contributed equally to this work. Correspondence to: Dr. med. Amin Polzin, PD. Klinik für Kardiologie, Pneumologie und Angiologie, Moorenstrasse 5, 40225 Düsseldorf, Germany. Email: [email protected]. Abstract: Target temperature management is recommended in post-resuscitation care. Additionally, hypothermia is a promising option in adjunctive therapy of acute myocardial infarction (MI). However, first in men data are contradicting. There are still many open questions to identify the optimal regimen and target temperature. In this study, we aimed to investigate the effect of very mild hypothermia on infarct size (IS) in mice. Mice underwent cardiac ischemia by temporary occlusion of the left anterior descending (LAD) artery under conditions of very mild hypothermia (34–36 ). Hypothermia was reached within the first 5 minutes of ischemia (temperature: 34.6±0.5 vs. 36.8±1.1 , P=0.035). Very mild hypothermia reduced IS ℃ in mice undergoing 30 minutes ischemia [IS/area at risk (AAR): 45±12% vs. 22±4%, P=0.018] as well as mice ℃ undergoing 60 minutes ischemia [IS/AAR: 67±7% vs. -
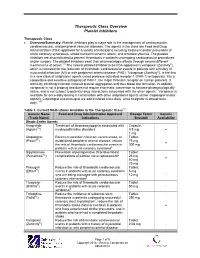
Therapeutic Class Overview Platelet Inhibitors
Therapeutic Class Overview Platelet Inhibitors Therapeutic Class • Overview/Summary: Platelet inhibitors play a major role in the management of cardiovascular, cerebrovascular, and peripheral vascular diseases. The agents in the class are Food and Drug Administration (FDA)-approved for a variety of indications including treatment and/or prevention of acute coronary syndromes, stroke/transient ischemic attack, and thrombocythemia. The platelet inhibitors are also indicated to prevent thrombosis in patients undergoing cardiovascular procedures and/or surgery. The platelet inhibitors exert their pharmacologic effects through several different mechanisms of action.1-8 The newest platelet inhibitor to be FDA-approved is vorapaxar (Zontivity®), which is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease (PAD).7 Vorapaxar (Zontivity®), is the first in a new class of antiplatelet agents called protease-activated receptor-1 (PAR-1) antagonists. It is a competitive and selective antagonist of PAR-1, the major thrombin receptor on human platelets. It works by inhibiting thrombin-induced platelet aggregation and thus blood clot formation. In addition, vorapaxar is not a prodrug and does not require enzymatic conversion to become pharmacologically active, and is not subject to potential drug interactions associated with the other agents.7 Vorapaxar is available for once-daily dosing in combination with other antiplatelet agents (either clopidogrel