Expression of Dual Nucleotidescysteinylleukotrienes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cangrelor Ameliorates CLP-Induced Pulmonary Injury in Sepsis By
Luo et al. Eur J Med Res (2021) 26:70 https://doi.org/10.1186/s40001-021-00536-4 European Journal of Medical Research RESEARCH Open Access Cangrelor ameliorates CLP-induced pulmonary injury in sepsis by inhibiting GPR17 Qiancheng Luo1†, Rui Liu2†, Kaili Qu3, Guorong Liu1, Min Hang1, Guo Chen1, Lei Xu1, Qinqin Jin1 , Dongfeng Guo1* and Qi Kang1* Abstract Background: Sepsis is a common complication of severe wound injury and infection, with a very high mortality rate. The P2Y12 receptor inhibitor, cangrelor, is an antagonist anti-platelet drug. Methods: In our study, we investigated the protective mechanisms of cangrelor in CLP-induced pulmonary injury in sepsis, using C57BL/6 mouse models. Results: TdT-mediated dUTP Nick-End Labeling (TUNEL) and Masson staining showed that apoptosis and fbrosis in lungs were alleviated by cangrelor treatment. Cangrelor signifcantly promoted surface expression of CD40L on platelets and inhibited CLP-induced neutrophils in Bronchoalveolar lavage fuid (BALF) (p < 0.001). We also found that cangrelor decreased the infammatory response in the CLP mouse model and inhibited the expression of infamma- tory cytokines, IL-1β (p < 0.01), IL-6 (p < 0.05), and TNF-α (p < 0.001). Western blotting and RT-PCR showed that cangre- lor inhibited the increased levels of G-protein-coupled receptor 17 (GPR17) induced by CLP (p < 0.001). Conclusion: Our study indicated that cangrelor repressed the levels of GPR17, followed by a decrease in the infam- matory response and a rise of neutrophils in BALF, potentially reversing CLP-mediated pulmonary injury during sepsis. Keywords: Sepsis, Infammation, Cangrelor, Platelet, GPR17 Background Te lung is one of the initial target organ of the systemic Sepsis is a serious disease and will lead a high mortal- infammatory response caused by sepsis, leading to alve- ity rate of approximately 22% in all over the world [1]. -

Kengrexal, INN-Cangrelor Tetrasodium
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Kengrexal 50 mg powder for concentrate for solution for injection/infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains cangrelor tetrasodium corresponding to 50 mg cangrelor. After reconstitution 1 mL of concentrate contains 10 mg cangrelor. After dilution 1 mL of solution contains 200 micrograms cangrelor. Excipient with known effect Each vial contains 52.2 mg sorbitol. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for injection/infusion. White to off-white lyophilised powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable. 4.2 Posology and method of administration Kengrexal should be administered by a physician experienced in either acute coronary care or in coronary intervention procedures and is intended for specialised use in an acute and hospital setting. Posology The recommended dose of Kengrexal for patients undergoing PCI is a 30 micrograms/kg intravenous bolus followed immediately by 4 micrograms/kg/min intravenous infusion. The bolus and infusion should be initiated prior to the procedure and continued for at least two hours or for the duration of the procedure, whichever is longer. At the discretion of the physician, the infusion may be continued for a total duration of four hours, see section 5.1. -

P2 Receptors in Cardiovascular Regulation and Disease
Purinergic Signalling (2008) 4:1–20 DOI 10.1007/s11302-007-9078-7 REVIEW P2 receptors in cardiovascular regulation and disease David Erlinge & Geoffrey Burnstock Received: 3 May 2007 /Accepted: 22 August 2007 /Published online: 21 September 2007 # Springer Science + Business Media B.V. 2007 Abstract The role of ATP as an extracellular signalling Introduction molecule is now well established and evidence is accumulating that ATP and other nucleotides (ADP, UTP and UDP) play Ever since the first proposition of cell surface receptors for important roles in cardiovascular physiology and pathophysi- nucleotides [1, 2], it has become increasingly clear that, in ology, acting via P2X (ion channel) and P2Y (G protein- addition to functioning as an intracellular energy source, the coupled) receptors. In this article we consider the dual role of purines and pyrimidines ATP, adenosine diphosphate ATP in regulation of vascular tone, released as a cotransmitter (ADP), uridine triphosphate (UTP) and uridine diphosphate from sympathetic nerves or released in the vascular lumen in (UDP) can serve as important extracellular signalling response to changes in blood flow and hypoxia. Further, molecules [3, 4] acting on 13 P2X homo- and heteromul- purinergic long-term trophic and inflammatory signalling is timer ionotropic and 8 P2Y metabotropic receptor subtypes described in cell proliferation, differentiation, migration and [5, 6] (Table 1). To terminate signalling, ectonucleotidases death in angiogenesis, vascular remodelling, restenosis and are present in the circulation and on cell surfaces, rapidly atherosclerosis. The effects on haemostasis and cardiac degrading extracellular ATP into ADP, AMP and adenosine regulation is reviewed. The involvement of ATP in vascular [7, 8]. -

A Comparative Study of Molecular Structure, Pka, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs
International Journal of Molecular Sciences Article A Comparative Study of Molecular Structure, pKa, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs Milan Remko 1,*, Anna Remková 2 and Ria Broer 3 1 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Comenius University in Bratislava, Odbojarov 10, SK-832 32 Bratislava, Slovakia 2 Department of Internal Medicine, Faculty of Medicine, Slovak Medical University, Limbová 12, SK–833 03 Bratislava, Slovakia; [email protected] 3 Department of Theoretical Chemistry, Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +421-2-5011-7291 Academic Editor: Michael Henein Received: 18 February 2016; Accepted: 11 March 2016; Published: 19 March 2016 Abstract: Theoretical chemistry methods have been used to study the molecular properties of antiplatelet agents (ticlopidine, clopidogrel, prasugrel, elinogrel, ticagrelor and cangrelor) and several thiol-containing active metabolites. The geometries and energies of most stable conformers of these drugs have been computed at the Becke3LYP/6-311++G(d,p) level of density functional theory. Computed dissociation constants show that the active metabolites of prodrugs (ticlopidine, clopidogrel and prasugrel) and drugs elinogrel and cangrelor are completely ionized at pH 7.4. Both ticagrelor and its active metabolite are present at pH = 7.4 in neutral undissociated form. The thienopyridine prodrugs ticlopidine, clopidogrel and prasugrel are lipophilic and insoluble in water. Their lipophilicity is very high (about 2.5–3.5 logP values). The polar surface area, with regard to the structurally-heterogeneous character of these antiplatelet drugs, is from very large interval of values of 3–255 Å2. -

Practical Guidance for the Evaluation and Management of Drug Hypersensitivity: Specific Drugs
Specific Drugs Practical Guidance for the Evaluation and Management of Drug Hypersensitivity: Specific Drugs Chief Editors: Ana Dioun Broyles, MD, Aleena Banerji, MD, and Mariana Castells, MD, PhD Ana Dioun Broyles, MDa, Aleena Banerji, MDb, Sara Barmettler, MDc, Catherine M. Biggs, MDd, Kimberly Blumenthal, MDe, Patrick J. Brennan, MD, PhDf, Rebecca G. Breslow, MDg, Knut Brockow, MDh, Kathleen M. Buchheit, MDi, Katherine N. Cahill, MDj, Josefina Cernadas, MD, iPhDk, Anca Mirela Chiriac, MDl, Elena Crestani, MD, MSm, Pascal Demoly, MD, PhDn, Pascale Dewachter, MD, PhDo, Meredith Dilley, MDp, Jocelyn R. Farmer, MD, PhDq, Dinah Foer, MDr, Ari J. Fried, MDs, Sarah L. Garon, MDt, Matthew P. Giannetti, MDu, David L. Hepner, MD, MPHv, David I. Hong, MDw, Joyce T. Hsu, MDx, Parul H. Kothari, MDy, Timothy Kyin, MDz, Timothy Lax, MDaa, Min Jung Lee, MDbb, Kathleen Lee-Sarwar, MD, MScc, Anne Liu, MDdd, Stephanie Logsdon, MDee, Margee Louisias, MD, MPHff, Andrew MacGinnitie, MD, PhDgg, Michelle Maciag, MDhh, Samantha Minnicozzi, MDii, Allison E. Norton, MDjj, Iris M. Otani, MDkk, Miguel Park, MDll, Sarita Patil, MDmm, Elizabeth J. Phillips, MDnn, Matthieu Picard, MDoo, Craig D. Platt, MD, PhDpp, Rima Rachid, MDqq, Tito Rodriguez, MDrr, Antonino Romano, MDss, Cosby A. Stone, Jr., MD, MPHtt, Maria Jose Torres, MD, PhDuu, Miriam Verdú,MDvv, Alberta L. Wang, MDww, Paige Wickner, MDxx, Anna R. Wolfson, MDyy, Johnson T. Wong, MDzz, Christina Yee, MD, PhDaaa, Joseph Zhou, MD, PhDbbb, and Mariana Castells, MD, PhDccc Boston, Mass; Vancouver and Montreal, -

Role of P2y Receptors in the Spinal-Trigeminal System in Vivo and in Vitro
UNIVERSITÀ DEGLI STUDI DI MILANO Facoltà di Farmacia Dipartimento di Scienze Farmacologiche Corso di Dottorato di Ricerca in Scienze Farmacotossicologiche, Farmacognostiche e Biotecnologie Farmacologiche (XXIII CICLO) Graduate School in Pharmacological Sciences / Scuola di Dottorato in Scienze farmacologiche TESI DI DOTTORATO DI RICERCA PURINERGIC TRANSMISSION IN MIGRAINE: ROLE OF P2Y RECEPTORS IN THE SPINAL-TRIGEMINAL SYSTEM IN VIVO AND IN VITRO BIO/14 Tesi di dottorato di: GIOVANNI VILLA MATRICOLA: R07517 TUTOR: Chiar.ma Prof.ssa Maria Pia ABBRACCHIO CORRELATORE: Dr.ssa Stefania CERUTI COORDINATORE: Chiar.mo Prof. Guido FRANCESCHINI ANNO ACCADEMICO 2009/2010 Index INDEX 1. INTRODUCTION _______________________________ 1 1.1 PAIN AND NOCICEPTION 2 1.1.1 Molecular basis of nociception 3 1.1.2 The trigeminal nerve and the spinal-trigeminal system 4 1.1.3 Role of non-neuronal cells in pain transmission 9 1.2 MIGRAINE 12 1.2.1 Description of the migraine attack 14 1.2.2 How and where does the migraine attack originate? 15 1.2.3 Familial hemiplegic migraine 21 1.2.4 Current and future pharmacological treatment of migraine 24 1.3 THE PURINERGIC SYSTEM 29 1.3.1 Purinergic signalling 30 1.3.2 P2X receptors 32 1.3.3 P2Y receptors 33 1.3.4 Pathophysiological roles of extracellular nucleotides in the nervous system 36 1.4 PURINES AND PAIN 41 1.4.1 Role of P2X receptors in pain transmission 42 1.4.2 Role of P2Y receptors in pain transmission: sensory ganglia 46 1.4.3 Role of P2Y receptors in pain transmission: CNS 48 2. -

Advances in Interventional Cardiology New Directions In
Advances in Interventional Cardiology New Directions in Antiplatelet Therapy Jose´ Luis Ferreiro, MD; Dominick J. Angiolillo, MD, PhD therosclerosis is a chronic inflammatory process that is A2 (TXA2) from arachidonic acid through selective acetylation Aknown to be the underlying cause of coronary artery of a serine residue at position 529 (Ser529). TXA2 causes disease (CAD).1 In addition to being the first step of primary changes in platelet shape and enhances recruitment and aggre- hemostasis, platelets play a pivotal role in the thrombotic gation of platelets through its binding to thromboxane and process that follows rupture, fissure, or erosion of an athero- prostaglandin endoperoxide (TP) receptors. Therefore, aspirin sclerotic plaque.2 Because atherothrombotic events are essen- decreases platelet activation and aggregation processes mediated tially platelet-driven processes, this underscores the impor- by TP receptor pathways.7 tance of antiplatelet agents, which represent the cornerstone Although the optimal dose of aspirin has been the subject of treatment, particularly in the settings of patients with acute of debate, the efficacy of low-dose aspirin is supported by the coronary syndromes (ACS) and undergoing percutaneous results of numerous studies.8–10 In these investigations, a coronary intervention (PCI). dose-dependent risk for bleeding, particularly upper gastro- Currently, there are 3 different classes of antiplatelet drugs that are approved for clinical use and recommended per intestinal bleeding, with no increase in -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

2020 Aetna Standard Plan
Plan for your best health Aetna Standard Plan Aetna.com Aetna is the brand name used for products and services provided by one or more of the Aetna group of subsidiary companies, including Aetna Life Insurance Company and its affiliates (Aetna). Aetna Pharmacy Management refers to an internal business unit of Aetna Health Management, LLC. Aetna Pharmacy Management administers, but does not offer, insure or otherwise underwrite the prescription drug benefits portion of your health plan and has no financial responsibility therefor. 2020 Pharmacy Drug Guide - Aetna Standard Plan Table of Contents INFORMATIONAL SECTION..................................................................................................................6 *ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS* - DRUGS FOR THE NERVOUS SYSTEM.................................................................................................................................16 *ALLERGENIC EXTRACTS/BIOLOGICALS MISC* - BIOLOGICAL AGENTS...............................18 *ALTERNATIVE MEDICINES* - VITAMINS AND MINERALS....................................................... 19 *AMEBICIDES* - DRUGS FOR INFECTIONS.....................................................................................19 *AMINOGLYCOSIDES* - DRUGS FOR INFECTIONS.......................................................................19 *ANALGESICS - ANTI-INFLAMMATORY* - DRUGS FOR PAIN AND FEVER............................19 *ANALGESICS - NONNARCOTIC* - DRUGS FOR PAIN AND FEVER......................................... -

Guideline/Protocol Title: GUIDELINES for the USE of ANTITHROMBOTIC AGENTS in the SETTING of NEURAXIAL PROCEDURES
Guideline/Protocol Title: GUIDELINES FOR THE USE OF ANTITHROMBOTIC AGENTS IN THE SETTING OF NEURAXIAL PROCEDURES Original Author(s): Matthias Behrends, MD; Ramana Naidu, MD; Margret Fang, MD, Christina Wang, PharmD; Daniel Burkhardt, MD; Erika Price, MD Collaborator(s): Ann Shah, MD, Chris Abrecht, MD; Tracy Minichiello, MD; Mark Schumacher, MD, PhD; Ashley Thompson, PharmD; Arthur Wood, MD; Pedram Aleshi, MD Approving committee(s): UCSF Health Pain Committee on 2/20/19, Antithrombotic and Hemostasis Committee on 5/25/19 Zuckerberg San Francisco General Hospital Pain Committee on 9/12/19, P&T on 9/27/19 P&T Approval Date: July 10th, 2019 (Parnassus) Last revision Date: Version 3.0, May 2015 PURPOSE/SCOPE: To allow the safe performance of neuraxial procedures in patients on antithrombotic medication. EXECUTIVE SUMMARY The intention of this guideline is to allow the safe performance of neuraxial procedures in patients on antithrombotic medication. Such neuraxial procedures include lumbar punctures, subarachnoid block (spinal), and the placement of intrathecal (e.g. lumbar drains) and epidural catheters. For high-risk neuraxial and other chronic pain procedures such as spinal cord stimulator or intrathecal delivery system implants, we advise providers to follow the guidelines of their specialty societies (Narouze et al, RAPM 2018). BACKGROUND / INTRODUCTION The primary concern for neuraxial procedures and anthithrombotics is the risk for epidural hematoma. The consequences of this complication include paralysis and permanent bowel/bladder dysfunction. The historic approximate risk of this complication is estimated to be 1 in 150,000 for epidurals and 1 in 220,000 for subarachnoid blocks (Bonica’s Management of Pain, 4th ed.) although evidence supports that the incidence may be as high as 1/9,000 for perioperative epidurals (MPOG- 2013). -

Download This PDF File
South African Family Practice 2019; 61(3):32-40 S Afr Fam Pract Open Access article distributed under the terms of the ISSN 2078-6190 EISSN 2078-6204 Creative Commons License [CC BY-NC-ND 4.0] © 2019 The Author(s) http://creativecommons.org/licenses/by-nc-nd/4.0 REVIEW To simplify long term To clot, or not to clot – Antithrombotic therapy is the question anti-platelet protection...switch to E Osuch,1 A Marais2 1 Department of Pharmacology and Therapeutics, School of Medicine, Sefako Makghato Health Sciences University, South Africa ® ® 2 Department of Pharmacology, School of Medicine, Faculty of Health Sciences, University of Pretoria, South Africa w s 75/75s 75/75 Corresponding author: [email protected] / [email protected] w Clopidogrel/Aspirin Haemostasis and thrombosis rely on three components namely the vascular endothelial wall, blood platelets and the coagulation cascade. Non-physiologic excessive thrombosis occurs when haemostatic processes are dysfunctional, causing undue clot Dual Protection – Today – Tomorrow formation or reduced clot lysis. Antithrombotic agents including antiplatelet, anticoagulation and fibrinolytic agents are essential Dual Protection – Today – Tomorrow for the prophylaxis and pharmacological management of venous thromboembolism and arterial thrombosis. Anticoagulation treatment options have expanded steadily over the past few decades, providing a greater number of agents. Anticoagulants that directly target the enzymatic activity of thrombin and factor Xa have recently been developed to address the inadequacies of traditional vitamin K antagonists. Appropriate use of these agents requires knowledge of their individual characteristics, risks, and benefits. For full prescriber information, please refer to the professional information approved by the medicine regulatory authority. -
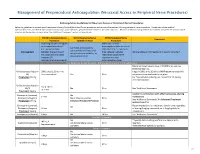
Neuraxial Or Nerve Procedures
Management of Periprocedural Anticoagulation (Neuraxial Access or Peripheral Nerve Procedures) Anticoagulation Guidelines for Neuraxial Access or Peripheral Nerve Procedures Below are guidelines to prevent spinal hematoma following Epidural/Intrathecal/Spinal procedures and perineural hematoma following peripheral nerve procedures. Procedures include epidural injections/infusions, intrathecal injections/infusions/pumps, spinal injections, peripheral nerve catheters, and plexus infusions. Decisions to deviate from guideline recommendations given the specific clinical situation are the decision of the provider. See ‘Additional Comments’ section for more details. PRIOR to Neuraxial/Nerve WHILE Neuraxial/Nerve AFTER Neuraxial/Nerve Comments Procedure Catheter in Place Procedure How long should I hold prior When can I restart to neuraxial procedure? anticoagulants after neuraxial Can I give anticoagulants (i.e., minimum time procedures? (i.e., minimum concurrently with neuraxial, Anticoagulant between the last dose of time between catheter What additional information do I need to consider? peripheral nerve catheter, or anticoagulant and spinal removal or spinal/nerve plexus placement? injection OR injection and next neuraxial/nerve placement) anticoagulation dose) Low-Molecular Weight Heparins, Unfractionated Heparin, and Fondaparinux Maximum total heparin dose of 10,000 units per day (5000 SQ Q12 hrs) Unfractionated Heparin 5000 units Q 12 hrs – no Heparin 5000 units SQ 8 hrs is NOT recommended with SQ time restrictions Yes 2 hrs concurrent