Fetal Monitoring and Umbilical Cord Gases: What's the Secret?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Versus Laboratory for Estimating of Dehydration Severity
Clinical versus laboratory for estimating of dehydration severity Majid Malaki Pediatric Health Research Center, Tabriz Medical University, Tabriz, Iran ABSTRACT Background: Acute gastroenteritis is a common cause of dehydration and precise estimation of dehydration Materials and Methods: D is a vital matter for clinical decisions. We try to find how much clinically diagnosed scales are compatible with ORIGINAL ARTICLE laboratory tests measures. uring 2 years 95 infants and children aged between 2 and 108 months entered to emergency room with acute gastroenteritis. They were categorized as mild, moderate and severe dehydration, their recorded laboratory tests include blood urea nitrogen (BUN), creatinine, venous blood gases values were expressedP by means ±95% of confidence intervalResult and compared by mann-whitney test in each groups with SPSS 16, sensitivity, specificity and likelihood ratio measured for defined cut off values in severe dehydration group, value less than 0.05 was significant. : Severe dehydration includes 3% Conclusionof all hospitalization: R due to dehydration. Laboratory tests cannot differentiate mild to moderate dehydration definietly but this difference is significant between severe to mild and severe to moderate dehydration. outine laboratory test are not generally helpful for dehydration severity estimation but they can be discriminate severe from mild or moderate dehydration exclusively. Creatinine higher than 0.9 mg/dl and BaseKey words deficit: beyond-16A are specific (90%) for severe dehydration estimation -

Severe Metabolic Acidosis in a Patient with an Extreme Hyperglycaemic Hyperosmolar State: How to Manage? Marloes B
Clinical Case Reports and Reviews Case Study ISSN: 2059-0393 Severe metabolic acidosis in a patient with an extreme hyperglycaemic hyperosmolar state: how to manage? Marloes B. Haak, Susanne van Santen and Johannes G. van der Hoeven* Department of Intensive Care Medicine, Radboud University Medical Center, Nijmegen, the Netherlands Abstract Hyperglycaemic hyperosmolar state (HHS) and diabetic ketoacidosis (DKA) are often accompanied by severe metabolic and electrolyte disorders. Analysis and treatment of these disorders can be challenging for clinicians. In this paper, we aimed to discuss the most important steps and pitfalls in analyzing and treating a case with extreme metabolic disarrangements as a consequence of an HHS. Electrolyte disturbances due to fluid shifts and water deficits may result in potentially dangerous hypernatriema and hyperosmolality. In addition, acid-base disorders often co-occur and several approaches have been advocated to assess the acid-base disorder by integration of the principles of mass balance and electroneutrality. Based on the case vignette, four explanatory methods are discussed: the traditional bicarbonate-centered method of Henderson-Hasselbalch, the strong ion model of Stewart, and its modifications ‘Stewart at the bedside’ by Magder and the simplified Fencl-Stewart approach. The four methods were compared and tested for their bedside usefulness. All approaches gave good insight in the metabolic disarrangements of the presented case. However, we found the traditional method of Henderson-Hasselbalch and ‘Stewart at the bedside’ by Magder most explanatory and practical to guide treatment of the electrolyte disturbances and in exploring the acid-base disorder of the presented case. Introduction This is accompanied by changes in pCO2 and bicarbonate (HCO₃ ) levels, depending on the cause of the acid-base disorder. -

Messages from the Placentae Across Multiple Species a 50 Years
Placenta 84 (2019) 14–27 Contents lists available at ScienceDirect Placenta journal homepage: www.elsevier.com/locate/placenta Messages from the placentae across multiple species: A 50 years exploration T Hiroaki Soma Saitama Medical University, Japan ARTICLE INFO ABSTRACT Keywords: This review explores eight aspects of placentation in multiple mammalian. Gestational trophoblastic disease 1) Specialities of gestational trophoblastic disease. SUA(Single umbilical artery) 2) Clinical significance of single umbilical artery (SUA) syndrome. DIC(Disseminated intravascular coagulation) in 3) Pulmonary trophoblast embolism in pregnant chinchillas and DIC in pregnant giant panda. giant panda 4) Genetics status and placental behaviors during Japanese serow and related antelopes. Placentation in Japanese serow 5) Specific living style and placentation of the Sloth and Proboscis monkey. Hydatidiform mole in chimpanzee Placentation in different living elephant 6) Similarities of placental structures between human and great apes. Manatee and hyrax 7) Similarities of placental forms in elephants, manatees and rock hyrax with different living styles. Specific placental findings of Himalayan people 8) Specialities of placental pathology in Himalayan mountain people. Conclusions: It was taught that every mammalian species held on placental forms applied to different environ- mental life for their infants, even though their gestational lengths were different. 1. Introduction of effective chemotherapeutic agents. In 1959, I was fortunate tore- ceive an invitation from Prof. Kurt Benirschke at the Boston Lying-in Last October, Scientific American published a special issue about a Hospital. Before that, I had written to Prof. Arthur T. Hertig, Chairman baby's first organ, the placenta [1]. It is full of surprises and amazing of Pathology, Harvard Medical School, asking to study human tropho- science. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -

Study on Acid-Base Balance Disorders and the Relationship
ArchiveNephro-Urol of Mon SID. 2020 May; 12(2):e103567. doi: 10.5812/numonthly.103567. Published online 2020 May 23. Research Article Study on Acid-Base Balance Disorders and the Relationship Between Its Parameters and Creatinine Clearance in Patients with Chronic Renal Failure Tran Pham Van 1, *, Thang Le Viet 2, Minh Hoang Thi 1, Lan Dam Thi Phuong 1, Hang Ho Thi 1, Binh Pham Thai 3, Giang Nguyen Thi Quynh 3, Diep Nong Van 4, Sang Vuong Dai 5 and Hop Vu Minh 1 1Biochemistry Department, Military Hospital 103, Hanoi, Vietnam 2Nephrology and Hemodialysis Department, Military Hospital 103, Hanoi, Vietnam 3National Hospital of Endocrinology, Hanoi, Vietnam 4Biochemistry Department, Backan Hospital, Vietnam 5Biochemistry Department, Thanh Nhan Hospital, Hanoi, Vietnam *Corresponding author: Biochemistry Department, Military Hospital 103, Hanoi, Vietnam. Email: [email protected] Received 2020 May 09; Accepted 2020 May 09. Abstract Objectives: We aimed to determine the parameters of acid-base balance in patients with chronic renal failure (CRF) and the rela- tionship between the parameters evaluating acid-base balance and creatinine clearance. Methods: The current cross-sectional study was conducted on 300 patients with CRF (180 males and 120 females). Clinical examina- tion and blood tests by taking an arterial blood sample for blood gas measurement as well as venous blood for biochemical tests to select study participants were performed. Results: Patients with CRF in the metabolic acidosis group accounted for 74%, other types of disorders were less common. The average pH, PCO2, HCO3, tCO2 and BE of the patient group were 7.35 ± 0.09, 34.28 ± 6.92 mmHg, 20.18 ± 6.06 mmol/L, 21.47 ± 6.48 mmHg and -4.72 ± 6.61 mmol/L respectively. -
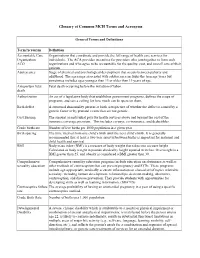
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

A Practical Approach to Acid-Base Balance for Small Animal Practitioners
A PRACTICAL APPROACH TO ACID-BASE BALANCE FOR SMALL ANIMAL PRACTITIONERS IVMA CE Self-Study Offering Dr Nicola Parry, DipACVP Midwest Veterinary Pathology, LLC Lafayette, IN AIM OF THIS ARTICLE Acid-base balance (ABB) is a convoluted concept that requires detailed comprehension of the metabolic pathways used to eliminate the H+ ion from the body. Not surprisingly, many practitioners find it daunting to retain the key concepts and apply them in a meaningful way clinical practice. This article aims to review some of the major points about ABB, and to provide a stepwise approach to evaluating laboratory data in order to identify key aspects of acid-base disorders. Although the chemical/biochemical basis of ABB is important, this article aims to share a practical and more factual, informal approach to the subject that will hopefully appeal to the majority of practitioners. Readers who crave extensive derivations of the Henderson-Hasselbalch equation are welcome to revisit their dusty textbooks for increased levels of excitement! LEARNING OBJECTIVES Following completion of this continuing education article, you will be able to: Indicate whether the pH level indicates acidosis or alkalosis List major sources of acids in the body Identify the major chemical buffer systems in the body Identify the cause of the pH imbalance as either respiratory or metabolic Distinguish between acidosis and alkalosis resulting from respiratory and metabolic factors Describe the importance of respiratory and renal compensations to ABB Determine if there is any compensation for the acid-base imbalance Identify the causes of high anion gap metabolic acidosis Use a systematic, step-by-step approach to diagnose acid-base disorders from laboratory data SO WHAT IS ABB & WHY DO WE CARE ABOUT IT? ABB is fundamental to physiologic homeostasis and refers to the way in which the body maintains a relatively constant pH despite continuous production of metabolic end products. -

BLOOD GAS ANALYSIS Deorari , AIIMS 2008
Deorari , AIIMS 2008 BLOOD GAS ANALYSIS Deorari , AIIMS 2008 Contents 1. Introduction, indications and sources of errors 2. Terminology and normal arterial blood gases 3. Understanding the print outs 4. Details about (i) pH (ii) Oxygenation, oxygen saturation, oxygen content, alveolar gas equation, indices of oxygenation (iii) Carbon dioxide transport, Pco2 total CO2 content, and bicarbonate levels (iv) Base excess and buffer base 5. Simple and mixed disorders 6. Compensation mechanisms 7. Anion Gap 8. Approach to arterial blood gases and exercises 9. Arterial blood gases decision tree 10. Practical tips for sampling for ABG. 2 Deorari , AIIMS 2008 Abbreviations ABE Actual base excess ABG Arterial blood gas AaDO2 Alveolar to arterial oxygen gradient Baro/PB Barometric pressure BB Buffer base BE Base excess BEecf Base excess in extracellular fluid BPD Bronchopulmonary dysplasia CH+ Concentration of hydrogen ion CO2 Carbon dioxide ECMO Extra corporeal membrane oxygenation FiO2 Fraction of inspired oxygen HCO3 Bicarbonate H2CO3 Carbonic acid MAP Mean airway pressure O2CT Oxygen content of blood PaCO2 Partial pressure of carbon dioxide in arterial blood PaO2 Partial pressure of oxygen in arterial blood pAO2 Partial pressure of oxygen in alveoli pH2O Water vapour pressure PPHN Persistent pulmonary hypertension in newborn RBC Red blood corpuscles 3 Deorari , AIIMS 2008 RQ Respiratory quotient Sat Saturation SBE Standard base excess - St HCO 3/SBC Standard bicarbonate TCO2 Total carbon dioxide content of blood THbA Total haemoglobin concentration UAC Umbilical artery catheter 4 Deorari , AIIMS 2008 The terminology of arterial blood gas (ABG) is complex and confusing. It is made worse by the printouts generated by recent microprocessors. -

Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG)
Lab Dept: Chemistry Test Name: VENOUS BLOOD GAS (VBG) General Information Lab Order Codes: VBG Synonyms: Venous blood gas CPT Codes: 82803 - Gases, blood, any combination of pH, pCO2, pO2, CO2, HCO3 (including calculated O2 saturation) Test Includes: VpH (no units), VpCO2 and VpO2 measured in mmHg, VsO2 and VO2AD measured in %, HCO3 and BE measured in mmol/L, Temperature (degrees C) and ST (specimen type) Logistics Test Indications: Useful for evaluating oxygen and carbon dioxide gas exchange; respiratory function, including hypoxia; and acid/base balance. It is also useful in assessment of asthma; chronic obstructive pulmonary disease and other types of lung disease; embolism, including fat embolism; and coronary artery disease. Lab Testing Sections: Chemistry Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 30 minutes Special Instructions: See Collection and Patient Preparation Specimen Specimen Type: Whole blood Container: Preferred: Sims Portex® syringe (PB151) or Smooth-E syringe (956- 463) Draw Volume: 0.4 mL (Minimum: 0.2 mL) blood Note: Submission of 0.2 mL of blood does not allow for repeat analysis. Processed Volume: 0.2 mL blood per analysis Collection: Avoid using a tourniquet. Anaerobically collect blood into a heparinized blood gas syringe (See Container. Once the puncture has been performed or the line specimen drawn, immediately remove all air from the syringe. Remove the needle, cap tightly and gently mix. Do not expose the specimen to air. Forward the specimen immediately at ambient temperature. Specimens cannot be stored. Note: When drawing from an indwelling catheter, the line must be thoroughly flushed with blood before drawing the sample. -

What Is the Role of the Placenta—Does It Protect Against Or Is It a Target for Insult?
Teratology Primer, 3rd Edition www.teratology.org/primer What Is the Role of the Placenta—Does It Protect Against or Is It a Target for Insult? Richard K. Miller University of Rochester School of Medicine & Dentistry Rochester, New York The placenta is not just a barrier but has many functions that are vital to the health of the embryo/fetus. The placenta is the anchor, the conduit, and the controller of pregnancy—and it can also be a target for toxicant action. The placenta encompasses not only the chorioallantoic placenta but all of its extraembryonic membranes (chorion/amnion) and the yolk sac. (Figure 1). The placenta and its membranes secure the embryo and fetus to the decidua (uterine lining) and release a variety of steroid and protein hormones that characterize the physiology of the pregnant female. Figure 1. Placental Structure in the Mouse. Figures depict early development of the mouse conceptus at embryonic days (E3.5, E7.5, E12.5). In the fetus, the visceral yolk sac (vYS) inverts and remains active throughout the entire gestation providing for transfer of selective large molecules, e.g., immunoglobulins IgG and vitamin B12. Abbreviations: Al, allantois; Am, amnion; Ch, chorion; Dec, decidua; Emb, embryo; Epc, ectoplacental cone; ICM, inner cell mass; Lab, Labyrinth; pYS, parietal yolk sac; SpT, spongiotrophoblast; TCG, trophoblast giant cell; Umb Cord, umbilical cord; vYS, visceral yolk sac; C-TGC, maternal blood canal trophoblast giant cell; P-TGC, parietal trophoblast giant cell; S-TGC, sinusoidal trophoblast giant cell; SpA-TGC, Spiral artery-associated trophoblast giant cell; Cyan-trophectoderm and trophoblast lineage, Black- inner cell mass and embryonic ectoderm; Gray -endoderm, Red-maternal vasculature, Purple-mesoderm, Yellow- decidua, Pink-fetal blood vessels in labyrinth. -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations. -

Postconditioning in Major Vascular Surgery: Prevention of Renal Failure
Aranyi et al. Journal of Translational Medicine (2015) 13:21 DOI 10.1186/s12967-014-0379-7 RESEARCH Open Access Postconditioning in major vascular surgery: prevention of renal failure Peter Aranyi1, Zsolt Turoczi1, David Garbaisz1, Gabor Lotz2, Janos Geleji3, Viktor Hegedus1, Zoltan Rakonczay4, Zsolt Balla4, Laszlo Harsanyi1 and Attila Szijarto1* Abstract Background: Postconditioning is a novel reperfusion technique to reduce ischemia-reperfusion injuries. The aim of the study was to investigate this method in an animal model of lower limb revascularization for purpose of preventing postoperative renal failure. Methods: Bilateral lower limb ischemia was induced in male Wistar rats for 3 hours by infrarenal aorta clamping under narcosis. Revascularization was allowed by declamping the aorta. Postconditioning (additional 10 sec reocclusion, 10 sec reperfusion in 6 cycles) was induced at the onset of revascularization. Myocyte injury and renal function changes were assessed 4, 24 and 72 hours postoperatively. Hemodynamic monitoring was performed by invasive arterial blood pressure registering and a kidney surface laser Doppler flowmeter. Results: Muscle viability studies showed no significant improvement with the use of postconditioning in terms of ischemic rhabdomyolysis (4 h: ischemia-reperfusion (IR) group: 42.93 ± 19.20% vs. postconditioned (PostC) group: 43.27 ± 27.13%). At the same time, renal functional laboratory tests and kidney myoglobin immunohistochemistry demonstrated significantly less expressed kidney injury in postconditioned animals (renal failure index: 4 h: IR: 2.37 ± 1.43 mM vs. PostC: 0.92 ± 0.32 mM; 24 h: IR: 1.53 ± 0.45 mM vs. PostC: 0.77 ± 0.34 mM; 72 h: IR: 1.51 ± 0.36 mM vs.