Abbreviations and Definitions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Messages from the Placentae Across Multiple Species a 50 Years
Placenta 84 (2019) 14–27 Contents lists available at ScienceDirect Placenta journal homepage: www.elsevier.com/locate/placenta Messages from the placentae across multiple species: A 50 years exploration T Hiroaki Soma Saitama Medical University, Japan ARTICLE INFO ABSTRACT Keywords: This review explores eight aspects of placentation in multiple mammalian. Gestational trophoblastic disease 1) Specialities of gestational trophoblastic disease. SUA(Single umbilical artery) 2) Clinical significance of single umbilical artery (SUA) syndrome. DIC(Disseminated intravascular coagulation) in 3) Pulmonary trophoblast embolism in pregnant chinchillas and DIC in pregnant giant panda. giant panda 4) Genetics status and placental behaviors during Japanese serow and related antelopes. Placentation in Japanese serow 5) Specific living style and placentation of the Sloth and Proboscis monkey. Hydatidiform mole in chimpanzee Placentation in different living elephant 6) Similarities of placental structures between human and great apes. Manatee and hyrax 7) Similarities of placental forms in elephants, manatees and rock hyrax with different living styles. Specific placental findings of Himalayan people 8) Specialities of placental pathology in Himalayan mountain people. Conclusions: It was taught that every mammalian species held on placental forms applied to different environ- mental life for their infants, even though their gestational lengths were different. 1. Introduction of effective chemotherapeutic agents. In 1959, I was fortunate tore- ceive an invitation from Prof. Kurt Benirschke at the Boston Lying-in Last October, Scientific American published a special issue about a Hospital. Before that, I had written to Prof. Arthur T. Hertig, Chairman baby's first organ, the placenta [1]. It is full of surprises and amazing of Pathology, Harvard Medical School, asking to study human tropho- science. -

FASD Effects of Alcohol on a Fetus
EFFECTS OF ALCOHOL ON A FETUS “Of all the substances of abuse (including cocaine, heroin, and marijuana), alcohol produces by far the most serious neurobehavioral effects in the fetus.” —Institute of Medicine Report to Congress, 19961 Prenatal exposure to alcohol can damage a fetus at any time, causing problems that persist throughout the individual’s life. There is no known safe level of alcohol use in pregnancy. WHAT IS THE SCOPE OF THE PROBLEM? identified in virtually every part of the body, including the brain, face, eyes, ears, heart, kidneys, and bones. No Alcohol is one of the most dangerous teratogens, which single mechanism can account for all the problems that are substances that can damage a developing fetus.1 Every alcohol causes. Rather, alcohol sets in motion many time a pregnant woman has a drink, her unborn child processes at different sites in the developing fetus: has one, too. Alcohol, like carbon monoxide from cigarettes, passes easily through the placenta from the • Alcohol can trigger cell death in a number of ways, mother's bloodstream into her baby's blood (See Figure causing different parts of the fetus to develop abnormally. 1)—and puts her fetus at risk of having a fetal alcohol • Alcohol can disrupt the way nerve cells develop, travel spectrum disorder (FASD). The blood alcohol level to form different parts of the brain, and function. (BAC) of the fetus becomes equal to or greater than the blood alcohol level of the mother. Because the fetus • By constricting the blood vessels, alcohol interferes with cannot break down alcohol the way an adult can, its BAC blood flow in the placenta, which hinders the delivery 2 remains high for a longer period of time. -
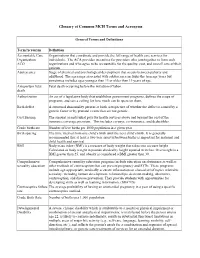
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

What Is the Role of the Placenta—Does It Protect Against Or Is It a Target for Insult?
Teratology Primer, 3rd Edition www.teratology.org/primer What Is the Role of the Placenta—Does It Protect Against or Is It a Target for Insult? Richard K. Miller University of Rochester School of Medicine & Dentistry Rochester, New York The placenta is not just a barrier but has many functions that are vital to the health of the embryo/fetus. The placenta is the anchor, the conduit, and the controller of pregnancy—and it can also be a target for toxicant action. The placenta encompasses not only the chorioallantoic placenta but all of its extraembryonic membranes (chorion/amnion) and the yolk sac. (Figure 1). The placenta and its membranes secure the embryo and fetus to the decidua (uterine lining) and release a variety of steroid and protein hormones that characterize the physiology of the pregnant female. Figure 1. Placental Structure in the Mouse. Figures depict early development of the mouse conceptus at embryonic days (E3.5, E7.5, E12.5). In the fetus, the visceral yolk sac (vYS) inverts and remains active throughout the entire gestation providing for transfer of selective large molecules, e.g., immunoglobulins IgG and vitamin B12. Abbreviations: Al, allantois; Am, amnion; Ch, chorion; Dec, decidua; Emb, embryo; Epc, ectoplacental cone; ICM, inner cell mass; Lab, Labyrinth; pYS, parietal yolk sac; SpT, spongiotrophoblast; TCG, trophoblast giant cell; Umb Cord, umbilical cord; vYS, visceral yolk sac; C-TGC, maternal blood canal trophoblast giant cell; P-TGC, parietal trophoblast giant cell; S-TGC, sinusoidal trophoblast giant cell; SpA-TGC, Spiral artery-associated trophoblast giant cell; Cyan-trophectoderm and trophoblast lineage, Black- inner cell mass and embryonic ectoderm; Gray -endoderm, Red-maternal vasculature, Purple-mesoderm, Yellow- decidua, Pink-fetal blood vessels in labyrinth. -

Equine Placenta – Marvelous Organ and a Lethal Weapon
Equine placenta – marvelous organ and a lethal weapon Malgorzata Pozor, DVM, PhD, Diplomate ACT Introduction Placenta has been defined as: „an apposition between parent (usually maternal) and fetal tissue in order to establish physiological exchange” (1). Another definition of this important organ was proposed by Steven and Morris: „a device consisting of one or more transport epithelia located between fetal and maternal blood supply” (2). The main function of placenta is to provide an interface between the dam and the the fetus and to allow the metabolic exchange of the the nutrients, oxygen and waste material. The maternal circulation is brought into a close apposition to the fetal circulation, while a separation of these two circulatory systems remain separated (3). A degree and complexity of this „intimate relationship” varies greately between species mostly due to the structural diversity of the extraembryonic membranes of the vertebrates. The early feto-maternal exchange in the equine pregnancy is established as early as on day 22 after fertilization. The fetal and choriovitellin circulations are already present, the capsule ruptures and the allantois is already visible (4). The allantois starts expanding by day 32 and vascularizes approximately 90% of the chorion and fuses with it to form chorioallantois by day 38 of gestation (5). The equine placenta continues increasing its complexity till approximately day 150 of gestation. Equids have epitheliochorial placenta, there are six leyers separating maternal and fetal circulation, and there are no erosion of the luminal, maternal epithelium, like in ruminants (6). Thousands of small chorionic microvilli develop and penetrate into endometrial invaginations. -

From Trophoblast to Human Placenta
From Trophoblast to Human Placenta (from The Encyclopedia of Reproduction) Harvey J. Kliman, M.D., Ph.D. Yale University School of Medicine I. Introduction II. Formation of the placenta III. Structure and function of the placenta IV. Complications of pregnancy related to trophoblasts and the placenta Glossary amnion the inner layer of the external membranes in direct contact with the amnionic fluid. chorion the outer layer of the external membranes composed of trophoblasts and extracellular matrix in direct contact with the uterus. chorionic plate the connective tissue that separates the amnionic fluid from the maternal blood on the fetal surface of the placenta. chorionic villous the final ramification of the fetal circulation within the placenta. cytotrophoblast a mononuclear cell which is the precursor cell of all other trophoblasts. decidua the transformed endometrium of pregnancy intervillous space the space in between the chorionic villi where the maternal blood circulates within the placenta invasive trophoblast the population of trophoblasts that leave the placenta, infiltrates the endo– and myometrium and penetrates the maternal spiral arteries, transforming them into low capacitance blood channels. Sunday, October 29, 2006 Page 1 of 19 From Trophoblasts to Human Placenta Harvey Kliman junctional trophoblast the specialized trophoblast that keep the placenta and external membranes attached to the uterus. spiral arteries the maternal arteries that travel through the myo– and endometrium which deliver blood to the placenta. syncytiotrophoblast the multinucleated trophoblast that forms the outer layer of the chorionic villi responsible for nutrient exchange and hormone production. I. Introduction The precursor cells of the human placenta—the trophoblasts—first appear four days after fertilization as the outer layer of cells of the blastocyst. -

Human Embryologyembryology
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology DevelopmentDevelopment inin FetalFetal PeriodPeriod 8.1 Characteristics of Fetal Period 210 days, from week 9 to delivery. characteristics: maturation of tissues and organs rapid growth of the body During 3-5 month, fetal growth in length is 5cm/M. In last 2 month, weight increases in 700g/M. relative slowdown in growth of the head compared with the rest of the body 8.2 Fetal AGE Fertilization age lasts 266 days, from the moment of fertilization to the day when the fetal is delivered. menstrual age last 280 days, from the first day of the last menstruation before pregnancy to the day when the fetal is delivered. The formula of expected date of delivery: year +1, month -3, day+7. ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FetalFetal membranesmembranes andand placentaplacenta Villous chorion placenta Decidua basalis Umbilical cord Afterbirth/ secundines Fusion of amnion, smooth chorion, Fetal decidua capsularis, membrane decidua parietalis 9.1 Fetal Membranes TheThe fetalfetal membranemembrane includesincludes chorionchorion,, amnion,amnion, yolkyolk sac,sac, allantoisallantois andand umbilicalumbilical cord,cord, originatingoriginating fromfrom blastula.blastula. TheyThey havehave functionsfunctions ofof protection,protection, nutrition,nutrition, respiration,respiration, excretion,excretion, andand producingproducing hormonehormone toto maintainmaintain thethe pregnancy.pregnancy. delivery 1) Chorion: villous and smooth chorion Villus chorionic plate primary villus trophoblast secondary villus extraembryonic tertiary villus mesoderm stem villus Amnion free villus decidua parietalis Free/termin al villus Stem/ancho chorion ring villus Villous chorion Smooth chorion Amniotic cavity Extraembyonic cavity disappears gradually; Amnion is added into chorionic plate; Villous and smooth chorion is formed. -

Human Trophoblast-Endometrial Interactions in an in Vitro Suspension Culture System
Placenta (I99o), i I, 349-367 Human Trophoblast-Endometrial Interactions in an In Vitro Suspension Culture System HARVEY J. KLIMAN a,c, RONALD F. FEINBERG b & JULIA E. HAIMOWITZ a ~ Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania J9io4, USA b Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19io4, USA ' To whom all correspondence should be addressed at: Department of Pathology and Laboratory Medicine, 6 Founders Pavilion, University of Pennsylvania School of Medicine, 34oo Spruce Street, Philadelphia, Pennsylvania i9zo4-4283, USA Paper accepted 19.3.z99o SUMMARY We developed an in vitro suspension co-culture system to examine the interaction of ist, 2nd and 3rd trimester purified cytotrophoblasts with human endometrium. Endometrium explants were added to cytotrophoblast cell suspensions and placed on an angled gyrating platform in a 37~ incubator. When endometrium was cultured alone it was able to remain viable for up to 3 days. When trophoblasts were cultured alone, they formed small and large aggregates, and occasionally spherical shells with hollow centers. When trophoblasts and endometrium were cultured together, the trophoblasts adhered to the exposed stromal surfaces of the tissue frag- ments. The surface epithelium was not receptive to trophoblast attachment except in one ex- periment when day ~9 endometrium was used for the co-incubation, suggesting that surface attachment is usually restricted. A common finding was the presence of an acellular zone in the endometrium only adjacent to the attached trophoblasts. We speculate that this zone may be caused by proteolysis and resynthesis of ECM proteins by the trophoblasts. -

Umbilical Cord Care N
n Umbilical Cord Care n (To keep your baby warm, baths should be quick during During pregnancy, the umbilical cord is the the first few weeks anyway.) baby’s connection to the mother. After birth, the cord is cut, and the stump remains connected to At every diaper change, use soap and water to clean the the baby. This is where the navel (belly button) will area around the cord stump. Pull up gently on the stump be. You’ll be taught some simple steps to care for and clean around its base. A little foul odor and moisture the cord stump after taking your baby home. are normal in this area. Although there are a few things to watch out for, Some doctors may recommend cleaning the stump with problems with the umbilical cord stump are rare. rubbing alcohol. You may clean the area with a small It usually falls off within a few weeks. amount of rubbing alcohol on a cotton ball or swab or use packaged alcohol wipes. Try not to use more rubbing alcohol than is necessary. Keep the cord stump over the diaper; it should not be What is the umbilical cord? inside the diaper. You may need to fold down the top While your baby was in the womb, he or she was edge of the diaper. connected to you by two important organs: the placenta and the umbilical cord. The umbilical cord is the fetus’s Are there any possible problems? “life support system” during pregnancy; it contains blood vessels that provide oxygen and nutrition. -

Human Development Summary
Human Development Laboratory Activity Gametogenesis Male The sperm develop within the highly coiled seminiferous tubules of the testes. When the sperm are fully mature they are extremely small, being little more than a bag of genetic material with a tail. The head of the sperm oell contains the nucleus and little else. The tail consists of a flagellum which allows the sperm to swim through the female reproductive tract to the egg. Female The egg is one of the largest cells in the body. As the egg ripens in the ovary it accumulates yolk which will serve as food for the young embryo until the placenta and umbilical cord are fully functional. Egg development is acomplished with the help of special nurse cells called follicle cells. When the egg is fully formed it bursts from the ovary in a process called ovulation. Ovulation is governed by complex nervous and hormonaI controls. After the egg is released from the ovary it enters the oviduct. Embryonic Development Fertilization Fertilization takes place in the fallopian tubes or oviducts. Although thousands of sperm cells may complete the trip to the egg only one will penetrate the cells outermost membrane and fertilize it. From this point on the egg is referred to as a zygote. Cleavage Divisions Almost as soon as the egg is fertilized it begins to divide. First into two cells, then 4, then 8 and so on. These divisions produce a solid clump of 32-64 cells called the morula. Blastocyst The morula continues its trip down the oviduct to the uterus. -

Fetal Monitoring and Umbilical Cord Gases: What's the Secret?
Fetal Monitoring and Umbilical Cord Gases: What’s the Secret? Patricia A. Heale, DNP, RNC-OB, C-EFM Objectives The learner will be able to describe the process for umbilical cord blood collection. define the normal acid-base parameters of umbilical cord blood gases. discuss the clinical value of determining acid-base parameters. What is Hypoxia? Low levels of oxygen in the tissues Acidemia & Acidosis Acidemia – state of low blood pH Acidosis – the process of becoming acidemic What is Asphyxia? Birth asphyxia occurs when a baby doesn't receive enough oxygen before, during or just after birth Hypoxia, hypercapnia, and respiratory and/or metabolic acidemia Fetal Asphyxia Occurrence ACOG reports that fetal asphyxia occurs in 25 of 1000 live births and 15% of those are moderate to severe Assessing Fetal Well-Being Ultrasound Biophysical Profile (BPP) Doppler studies Electronic Fetal Monitor Umbilical Cord Blood Analysis Electronic Fetal Monitor “…a valuable but imperfect tool.” (Clark, et al. 2017, p. 163) Expected Outcomes Decrease incidence of cerebral palsy and intrapartum stillbirth Actual Outcomes No difference # intrapartum stillbirth (one in 300) There were no differences in the incidence of CP Neonatal seizures decreased with EFM Continuous monitoring is associated with a significant increase in C/S and operative births Liability of EFM Year CD% 2015 32.0% 2007 31.8% 1996 20.7% 1988 24.7% 1970 5.5% Benefit Not Realized Unrealistically high expectations Lack of standardization of FHR definitions until 1997/2008 Poor reliability for FHR interpretation Failure to show the validity of FHR monitoring in detecting fetal asphyxia Electronic Fetal Monitor “…of infants born with metabolic acidemia, only approximately one-half could be identified potentially and have delivery expedited, even under ideal circumstances.” (Clark, et al, 2017, p. -

Alcohol Use in Pregnancy
Alcohol Use in Pregnancy Women who are pregnant or who may be pregnant should not drink alcohol. This includes: • women who are trying to get pregnant • women who are at risk of becoming pregnant because they do not use effective birth control (contraception). Why is alcohol use during pregnancy dangerous? Drinking alcohol during pregnancy can cause lifelong physical, behavioral, and intellectual disabilities. Alcohol in the mother's blood passes to the baby through the umbilical cord. Drinking alcohol during pregnancy can cause miscarriage, stillbirth, and a range of disabilities. These disabilities are known as Fetal Alcohol Spectrum Disorders (FASDs). Fetal alcohol syndrome (FAS) is the most complex Fetal Alcohol Spectrum Disorder. A baby born with FAS has a small head, weighs less than other babies, and has distinctive facial features. Some of the behavioral and intellectual disabilities of people with FASDs include: • Difficulty with learning or memory • Higher than normal level of activity (hyperactivity) • Difficulty with attention • Speech and language delays • Low IQ • Poor reasoning and judgment skills People born with FASDs can also have problems with their organs, including the heart and kidneys. FASDs are completely preventable if a woman does not drink alcohol during pregnancy. Why take the risk? Home Care Services - 1 - Home Care Services Alcohol Use in Pregnancy - 2 - If you are pregnant or trying to get pregnant and cannot stop drinking visit: • National Organization on Fetal Alcohol Syndrome (NOFAS): http://www.nofas.org Or call (800) 666 – 6327 Disclaimer: This document contains information and/or instructional materials developed by Michigan Medicine for the typical patient with your condition.