DNA Barcoding of Brycon Henni
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Evidence of a Spawning Site of the Endangered Fish Brycon
Short Research Note Acta Limnologica Brasiliensia, 2020, vol. 32, e23 https://doi.org/10.1590/S2179-975X2220 ISSN 2179-975X on-line version First evidence of a spawning site of the endangered fish Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) in the Middle Uruguay River, Brazil Primeira evidência de um local de desova do peixe ameaçado de extinção Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) no Médio rio Uruguai, Brasil David Augusto Reynalte-Tataje1* , Marlon da Luz Soares1 , Marthoni Vinicius Massaro1 , Rodrigo Bastian1 , Fernando Mayer Pelicice2 1 Universidade Federal da Fronteira Sul – UFFS. Rua Jacob Reinaldo Haupenthal, 1580, CEP 97900-000, Cerro Largo, RS, Brasil 2 Núcleo de Estudos Ambientais, Universidade Federal do Tocantins – UFT, Rua 3, Quadra 11 17, Jardim dos Ipês, CEP 77500-000, Porto Nacional, TO, Brasil *e-mail: [email protected] Cite as: Reynalte-Tataje, D.A. et al. First evidence of a spawning site of the endangered fish Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) in the Middle Uruguay River, Brazil. Acta Limnologica Brasiliensia, 2020, vol. 32, e23. Abstract: Aim: This study provides the first direct evidence about the location of a spawning site of Brycon orbignyanus (Valenciennes, 1850) in the Middle Uruguay River. Methods: Sampling was carried out with a plankton net (500 µm) in November 2017 in the Uruguay River near the confluence with the Piratinim River, southern Brazil. Eggs were sorted in the field, and to confirm species identity we used the live ichthyoplankton technique. Results: We captured 47 eggs of B. orbignyanus in the channel of the Uruguay River, characterized by fast flow, shallow depth (approximately 3 m) and rocky bottom, located a few kilometers upstream from its confluence with the Piratinim River. -

BMC Evolutionary Biology, 2014, 14
Abe et al. BMC Evolutionary Biology 2014, 14:152 http://www.biomedcentral.com/1471-2148/14/152 RESEARCH ARTICLE Open Access Systematic and historical biogeography of the Bryconidae (Ostariophysi: Characiformes) suggesting a new rearrangement of its genera and an old origin of Mesoamerican ichthyofauna Kelly T Abe, Tatiane C Mariguela, Gleisy S Avelino, Fausto Foresti and Claudio Oliveira* Abstract Background: Recent molecular hypotheses suggest that some traditional suprageneric taxa of Characiformes require revision, as they may not constitute monophyletic groups. This is the case for the Bryconidae. Various studies have proposed that this family (considered a subfamily by some authors) may be composed of different genera. However, until now, no phylogenetic study of all putative genera has been conducted. Results: In the present study, we analyzed 27 species (46 specimens) of all currently recognized genera of the Bryconidae (ingroup) and 208 species representing all other families and most genera of the Characiformes (outgroup). Five genes were sequenced: 16SrRNA, Cytochrome b, recombination activating gene 1 and 2 and myosin heavy chain 6 cardiac muscle. The final matrix contained 4699 bp and was analyzed by maximum likelihood, maximum parsimony and Bayesian analyses. The results show that the Bryconidae, composed of Brycon, Chilobrycon, Henochilus and Salminus, is monophyletic and is the sister group of Gasteropelecidae + Triportheidae. However, the genus Brycon is polyphyletic. Fossil studies suggest that the family originated approximately 47 million years ago (Ma) and that one of the two main lineages persisted only in trans-Andean rivers, including Central American rivers, suggesting a much older origin of Mesoamerican ichthyofauna than previously accepted. -

Genetic Structuring of Salminus Hilarii Valenciennes, 1850 (Teleostei
Neotropical Ichthyology, 13(3): 547-556, 2015 Copyright © 2015 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20150015 Genetic structuring of Salminus hilarii Valenciennes, 1850 (Teleostei: Characiformes) in the rio Paraná basin as revealed by microsatellite and mitochondrial DNA markers Juliana Viana da Silva1, Eric M. Hallerman2, Laura Helena Orfão3 and Alexandre Wagner Silva Hilsdorf1 Genetic variation of Salminus hilarii was assessed by screening microsatellite loci and mitochondrial D-loop DNA across four sampling in the upper rio Paraná basin of Brazil. Genetic diversity – measured as mean expected heterozygosity (0.904) and mean number of alleles across populations (13.7) – was reasonably high. Differentiation of microsatellite allele frequencies among populations was shown to be low but significant by AMOVAΦ ST (0.0192), and high by DEST (0.185). D-loop variation was high, with haplotypic diversity of 0.950 and nucleotide diversity of 0.011. Mitochondrial DNA-based estimates for population differentiation were high, with an overall ΦST of 0.173. The results of tests of nuclear and mitochondrial variation yielded no unequivocal inference of historical demographic bottleneck or expansion. Genetic differentiation observed among S. hilarii populations in the rio Grande may be caused by a combination of historical differentiation and recent gene-flow disruption caused by the dams followed by reproduction of isolated spawning assemblages in mid-sized tributaries of the respective reservoirs. We present spatially more intensive sampling of S. hilarii populations across the rio Paraná basin in order to more effectively distinguish between historical and contemporary differentiation. A variabilidade genética de Salminus hilarii foi avaliada por lócus microssatélites e sequências D-Loop do DNA mitocondrial em quatro populações da região da bacia do Alto Paraná. -

The Unique Karyotype of Henochilus Wheatlandii, a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil
The Unique Karyotype of Henochilus wheatlandii,a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil Priscilla C. Silva1*, Udson Santos1, Nata´lia M. Travenzoli1, Jose C. Zanuncio1, Marcelo de B. Cioffi2, Jorge A. Dergam1 1 Laborato´rio de Sistema´tica Molecular - Beagle, Departamento de Biologia Animal, Universidade Federal de Vic¸osa, Vic¸osa, Brazil, 2 Laborato´rio de biodiversidade molecular e citogene´tica, Departamento de Gene´tica e Evoluc¸a˜o, Universidade Federal de Sa˜o Carlos, Sa˜o Carlo, Brazil Abstract Henochilus wheatlandii, the only species of this genus, is critically endangered and was considered extinct for over a century. The rediscovery of this fish in 1996 made it possible to study its phylogenetic relationships with other species in the subfamily Bryconinae. The aim of this study was to characterise the karyotype of H. wheatlandii. Standard staining, C- positive heterochromatin and nucleolar organiser region (NOR) banding, chromomycin A3 staining, and fluorescent in situ hybridisation (FISH) using 5S rDNA and 18S rDNA probes were conducted on nineteen specimens collected in the Santo Antonio River, a sub-basin of the Doce River in Ferros municipality, Minas Gerais State, Brazil. Henochilus wheatlandii shared the same diploid number and chromosome morphology as other species of Bryconinae. However, its heterochromatin distribution patterns, NOR localisation, and FISH patterns revealed a cytogenetic profile unique among Neotropical Bryconinae, emphasizing the evolutionary uniqueness of this threatened species. Citation: Silva PC, Santos U, Travenzoli NM, Zanuncio JC, Cioffi MdB, et al. (2012) The Unique Karyotype of Henochilus wheatlandii, a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil. -
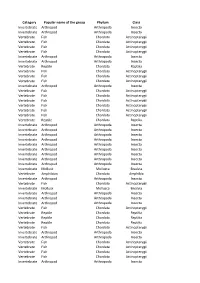
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Guía De Peces Para Aguas Continentales En La Vertiente Occidental Del Ecuador
GUÍA DE PecesPARA AGUAS CONTINENTALES EN LA VERTIENTE OCCIDENTAL DEL ECUADOR Pedro Jiménez Prado Windsor Aguirre Enrique Laaz Moncayo Ronald Navarrete Amaya Fredy Nugra Salazar Eduardo Rebolledo Monsalve Edwin Zárate Hugo Antonio Torres Noboa Jonathan Valdiviezo Rivera 2015 Fotografías: Antonio Torres Noboa Cesar Román Valencia Eduardo Rebolledo Monsalve Edwin Zárate Hugo Enrique Laaz Moncayo Fredy Nugra Salazar Javier Maldonado Ocampo Jonathan Valdiviezo Rivera Nathan Lujan Pedro Jiménez Prado Ronald Navarrte Amaya Windsor Aguirre Los textos pueden ser utilizados total o parcialmente citando la fuente. Citación sugerida: Jiménez-Prado, P., W. Aguirre, E. Laaz-Moncayo, R. Navarrete-Amaya, F. Nugra-Salazar, E. Rebolledo-Monsalve, E. Zárate-Hugo, A. Torres-Noboa y J. Valdiviezo-Rivera. (2015). Guía de peces para aguas continentales en la vertiente occidental del Ecuador. Pontificia Universidad Católica del Ecuador Sede Esmeraldas (PUCESE); Universidad del Azuay (UDA) y Museo Ecuatoriano de Ciencias Naturales (MECN) del Instituto Nacional de Biodiversidad. Esmeraldas, Ecuador. 416 pp. Responsabilidad: El material aquí presentado, así como las opiniones expresadas, no representan necesariamente el juicio o las políticas de las instituciones participantes. Todo lo expresado es de la entera responsabilidad de los autores. Los escritos y la información presentada han sido editadas con aprobación previa de sus autores. ISBN: 978-9942-20-740-1. D.R.® Pontificia Universidad Católica del Ecuador Sede Esmeraldas (2015). Diseño y diagramación: Miguel Dávila P. - Soluciones Gráficas D&G Cía. Ltda. Impresión: Imprenta Mariscal Cía. Ltda. Quito, Ecuador (Tiraje: 2.000 ejemplares) Con certificación Forest Stewardship Council, FSC. Corrección de estilo: Oswaldo Encalada Vásquez Con el apoyo de EcoFondo. El EcoFondo es un fondo ecológico fiduciario de carácter privado, que nace por decisión voluntaria de OCP Ecuador S.A. -

Conservation Status and Bio-Ecology of Brycon Orbignyanus
Neotropical Ichthyology, 17(3): e190030, 2019 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20190030 Published online: 24 October 2019 (ISSN 1982-0224) Copyright © 2019 Sociedade Brasileira de Ictiologia Printed: 30 September 2019 (ISSN 1679-6225) Original article Conservation status and bio-ecology of Brycon orbignyanus (Characiformes: Bryconidae), an endemic fish species from the Paraná River basin (Brazil) threatened with extinction Lívia Helena Tonella1, Rosa Maria Dias1,2, Oscar Barroso Vitorino Junior1, Rosemara Fugi1,2 and Angelo Antonio Agostinho1,2 Brycon orbignyanus is an endemic species from La Plata basin whose stocks have been presenting significant reductions throughout the Paraná River. Brycon orbignyanus is categorized as an endangered species. This study evaluated aspects of the bio-ecology of this species that may be related to this threat, highlighting its distribution, abundance, and diet as well as the corresponding relationships between its recruitment and flood regimes. Data were obtained from different parts of the upper Paraná River (stretches free and regulated by dams) from 1986 to 2010 with more detailed data collected from the free remnant of this basin. The results indicate that no records for species exist at more than half of the sampling points located in dam-regulated sections of the Paraná River, whereas specimens were collected from 75% sites in the free plain remnant. We observed a remarkable effect of the hydrological regime on recruitment as well as distinct food demands during ontogenetic development, with adults almost exclusively consuming fruits and seeds, revealing that these individuals are supported by riparian vegetation. Thus, it is concluded that changes in the natural flood regime as well as riparian vegetation removal threaten B. -

Reproductive Characteristics of Characid Fish Species (Teleostei
Reproductive characteristics of characid fish species (Teleostei... 469 Reproductive characteristics of characid fish species (Teleostei, Characiformes) and their relationship with body size and phylogeny Marco A. Azevedo Setor de Ictiologia, Museu de Ciências Naturais, Fundação Zoobotânica do Rio Grande do Sul, Rua Dr. Salvador França, 1427, 90690-000 Porto Alegre, RS, Brazil. ([email protected]) ABSTRACT. In this study, I investigated the reproductive biology of fish species from the family Characidae of the order Characiformes. I also investigated the relationship between reproductive biology and body weight and interpreted this relationship in a phylogenetic context. The results of the present study contribute to the understanding of the evolution of the reproductive strategies present in the species of this family. Most larger characid species and other characiforms exhibit a reproductive pattern that is generally characterized by a short seasonal reproductive period that lasts one to three months, between September and April. This is accompanied by total spawning, an extremely high fecundity, and, in many species, a reproductive migration. Many species with lower fecundity exhibit some form of parental care. Although reduction in body size may represent an adaptive advantage, it may also require evolutionary responses to new biological problems that arise. In terms of reproduction, smaller species have a tendency to reduce the number of oocytes that they produce. Many small characids have a reproductive pattern similar to that of larger characiforms. On the other hand they may also exhibit a range of modifications that possibly relate to the decrease in body size and the consequent reduction in fecundity. -

Brycon Orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae): Basis for Management Programs
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Portal de Revistas do ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) 18 Número misto: Conservação de Peixes Continentais e Manejo de Unidades de Conservação Conservation Status of the “Piracanjuba” Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae): Basis for Management Programs Daniela José de Oliveira¹, Fernando Yuldi Ashikaga¹, Fausto Foresti¹ & José Augusto Senhorini² Recebido em 28/05/2015 – Aceito em 07/04/2016 RESUMO – A exploração excessiva dos recursos de água doce tem causado muitos impactos sobre as populações naturais, especialmente em peixes. Diferentes fatores relacionados com a poluição, sobrepesca, introdução de espécies exóticas, construção de barragens e destruição de vegetação ripárias podem determinar mudanças nas populações atuais e interferir em seu equilíbrio. Neste contexto, Brycon orbignyanus, popularmente conhecido como “piracanjuba”, é uma espécie de peixe ameaçada de extinção pela pesca excessiva e degradação do meio ambiente. A qualidade da carne e do seu comportamento agressivo na prática da pesca desportiva e profissional, ocorrendo concomitantemente às mudanças contínuas no habitat, resultaram no esgotamento rápido dos estoques naturais e determinaram sua entrada na lista de espécies de peixes brasileiros ameaçados de extinção. A espécie tem distribuição ao longo da bacia do Rio da Prata em áreas altamente impactadas pela ocupação demográfica, construção de barragens e atividades agrícolas em áreas marginais. Populações nativas desta espécie ocupam regiões menos afetadas, sendo atualmente restrita a poucos rios distantes de centros urbanos e pequenos afluentes onde as condições ambientais permanecem preservadas. Com o objetivo de verificar o estado de conservação das populações de B. -

Phylogeny and Biogeography of Triportheidae (Teleostei: Characiformes) Based on Molecular Data Q ⇑ T.C
Molecular Phylogenetics and Evolution 96 (2016) 130–139 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny and biogeography of Triportheidae (Teleostei: Characiformes) based on molecular data q ⇑ T.C. Mariguela a, , F.F. Roxo b, F. Foresti b, C. Oliveira b a Departamento de Zoologia, Instituto de Biociências, Univ. Estadual Paulista – UNESP, Rio Claro, São Paulo, Brazil b Departamento de Morfologia, Instituto de Biociências, Univ. Estadual Paulista – UNESP, Botucatu, São Paulo, Brazil article info abstract Article history: Triportheidae represents a relatively small family of characiform fishes with highly modified morphol- Received 29 July 2015 ogy. The relationship among characiform families is still unclear, and a phylogenetic analysis for the Revised 13 November 2015 family including a representative number of Triportheus species has never been performed. Here, we Accepted 22 November 2015 inferred a phylogeny for 19 of the 22 species recognized for this family and two possible new Available online 22 December 2015 Triportheus species using two mitochondrial and three nuclear genes. Our results show that (1) Triportheidae is monophyletic and a sister group of the clade consisting of the families Bryconidae and Keywords: Gasteropelecidae; (2) Triportheus is monophyletic, but some species need to be reviewed and described; Evolution (3) all genera in Triportheidae, except for Agoniates originated in the period between Early Oligocene and Freshwater sardines Phylogeny Early Miocene; and (4) speciation in Triportheidae coincides with important geological events in South Molecular systematics America, reinforcing the importance of time-calibrated trees to study fish evolution. Neotropical fishes Ó 2015 Elsevier Inc. -

Fish Species
Fish Species Dorado or Golden Dorado- Classification: Kingdom: Animalia Phylum: Chordata Class: Actinopterygii Order: Characiformes Family: Characidae Genus: Salminus Species: maxillosus Binomial name: Salminus maxillosus Others: Salminus cuvieri, Salminus brasiliensis Characteristics: The dorado has been often referred to as the “tigre de rio” or jaguar of the river to be more accurate. They possess a bright golden coloration, accented by rows of tiny small back dots running along their powerful streamlined bodies. Their fins have a bright red coloration and the caudal fin has a black bar along the middle through the caudal peduncle. Their massive jaws are equipped with rows of razor sharp pointy teeth, living up quite accurately to its local nickname. Dorado can reach upwards of 100cm in length and weigh as much as 45lbs (20.5kgs), more commonly in the 22-33lb (10-15kg) range. Tabarana, the silver dorado- Classification- Kingdom: Animalia Phylum: Chordata Class: Actinopterygii Order: Characiformes Family: Characidae Genus: Salminus Species: hilarii Binomial name: Salminus hilarii Characteristics: This species is a close relative of the dorado, and shares many of its morphological features. However, this fish is smaller in average size, and has less girth. It is silver with bright orange specks between scales, and black and red accents on the caudal fin, as well as black accents on the dorsal and anal fins. This is why it is called the “white or silver dorado” in much of its range. This species is quite aggressive and preys upon smaller tetras and other baitfish such as sabalo (Prochilodus sp.). Its sharp teeth make quick work of the smaller characins in the river system. -

The Etyfish Project © Christopher Scharpf and Kenneth J
CHARACIFORMES (part 8) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 19.0 - 15 Sept. 2020 Order CHARACIFORMES (part 8 of 8) Family IGUANODECTIDAE 4 genera/subgenera · 38 species Bryconops Kner 1858 -ops, appearance, apparently reflecting Kner’s belief that genus could be “classified with the hither related species of the genus Brycon” (translation) Subgenus Bryconops Bryconops alburnoides Kner 1858 -oides, having the form of: the European cyprinid genus Alburnus Bryconops caudomaculatus (Günther 1864) caudo-, tail; maculatus, spotted, referring to pair of large reddish spots on basal half of caudal fin Bryconops collettei Chernoff & Machado-Allison 2005 in honor of Bruce B. Collette (b. 1935), Senior Systematic Zoologist, National Marine Fisheries Service, for “important” contributions to systematic ichthyology and to both author’s careers Bryconops disruptus Machado-Allison & Chernoff 1997 broken off, referring to incomplete lateral line Bryconops durbinae (Eigenmann 1908) in honor of Marion Durbin Ellis (née Durbin, 1887-1972), Eigenmann’s student and frequent collaborator in studying characid fishes, later a limnologist and environmental toxicologist, University of Missouri in Columbia Bryconops gracilis (Eigenmann 1908) slender, presumably referring to elongate, slightly compressed body Bryconops hexalepis Guedes, Oliveira & Lucinda 2019 hexa-, six; lepis, scale, referring to six scale rows above lateral line (most congeners have 7-10) Bryconops magoi Chernoff & Machado-Allison 2005 in memory of