Phylogeny and Biogeography of Triportheidae (Teleostei: Characiformes) Based on Molecular Data Q ⇑ T.C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estimating Migratory Fish Distribution from Altitude and Basin Area: a Case
Freshwater Biology (2012) 57, 2297–2305 doi:10.1111/fwb.12003 Estimating migratory fish distribution from altitude and basin area: a case study in a large Neotropical river JOSE´ RICARDO S. BARRADAS*, LUCAS G. SILVA*, BRET C. HARVEY† AND NELSON F. FONTOURA* *Departamento de Biodiversidade e Ecologia, Pontifı´cia Universidade Cato´lica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brazil †USDA Forest Service, Pacific Southwest Research Station, Arcata, CA, U.S.A. SUMMARY 1. The objective of this study was to identify longitudinal distribution patterns of large migratory fish species in the Uruguay River basin, southern Brazil, and construct statistical distribution models for Salminus brasiliensis, Prochilodus lineatus, Leporinus obtusidens and Pseudoplatystoma corruscans. 2. The sampling programme resulted in 202 interviews with old residents and fishermen at 167 different sampling sites in the major rivers of the basin. Interviewees were asked whether these fish species were present in the nearby river segment, recently or in the past. The average age of respondents was 56 years, and the great majority had resided for more than 30 years in the area where they were interviewed. 3. Fish distribution patterns were modelled using altitude and basin area as environmental descriptors. Distributional patterns were estimated using logistic equations (LOGIT): À1 P ¼ eða0þa1 lnðAlÞþa2 lnðBAÞÞð1 þ eða0þa1 lnðAlÞþa2 lnðBAÞÞÞ , where Al is the altitude and BA is the basin area. 4. Accuracies of the distribution models were between 77 and 85%. These accuracies are similar to those of published distribution models for other fishes based on larger numbers of environmental descriptors. 5. The historical distributional ranges make clear that all operating or planned large hydropower dams in the Uruguay River basin, with one exception, limit or will limit the distribution of migratory fishes. -

First Evidence of a Spawning Site of the Endangered Fish Brycon
Short Research Note Acta Limnologica Brasiliensia, 2020, vol. 32, e23 https://doi.org/10.1590/S2179-975X2220 ISSN 2179-975X on-line version First evidence of a spawning site of the endangered fish Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) in the Middle Uruguay River, Brazil Primeira evidência de um local de desova do peixe ameaçado de extinção Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) no Médio rio Uruguai, Brasil David Augusto Reynalte-Tataje1* , Marlon da Luz Soares1 , Marthoni Vinicius Massaro1 , Rodrigo Bastian1 , Fernando Mayer Pelicice2 1 Universidade Federal da Fronteira Sul – UFFS. Rua Jacob Reinaldo Haupenthal, 1580, CEP 97900-000, Cerro Largo, RS, Brasil 2 Núcleo de Estudos Ambientais, Universidade Federal do Tocantins – UFT, Rua 3, Quadra 11 17, Jardim dos Ipês, CEP 77500-000, Porto Nacional, TO, Brasil *e-mail: [email protected] Cite as: Reynalte-Tataje, D.A. et al. First evidence of a spawning site of the endangered fish Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae) in the Middle Uruguay River, Brazil. Acta Limnologica Brasiliensia, 2020, vol. 32, e23. Abstract: Aim: This study provides the first direct evidence about the location of a spawning site of Brycon orbignyanus (Valenciennes, 1850) in the Middle Uruguay River. Methods: Sampling was carried out with a plankton net (500 µm) in November 2017 in the Uruguay River near the confluence with the Piratinim River, southern Brazil. Eggs were sorted in the field, and to confirm species identity we used the live ichthyoplankton technique. Results: We captured 47 eggs of B. orbignyanus in the channel of the Uruguay River, characterized by fast flow, shallow depth (approximately 3 m) and rocky bottom, located a few kilometers upstream from its confluence with the Piratinim River. -

Aquaculture Production in Brazil 600000 562.533 500000
Transformations in Brazilian aquaculture: technological intensification and capital concentration Manoel Pedroza Embrapa Fisheries and Aquaculture Brazilian Agricultural Research Corporation What about the Brazilian aquaculture? » Significant growth in recent years Aquaculture production in Brazil 600000 562.533 500000 Tons 14º biggest - 400000 Inland 300000 world Marine 200000 producer 100000 Total Production Production 0 2008 2010 2012 2014 » Inland production semi-intensive and extensive systems Floating cages Earthen ponds Source: FAO, 2016 » Great diversity of species » Manly native » Geographical dispersion Aquaculture production in Brazil (2014) • White legged shrimp • Tilapia (42%) (O. niloticus) ≠’s strains (78%) (L.vannamei) (Thai/Chitralada/ Gift) 18% • Tambaqui (29%) (Colossoma macropomum) • Pacific oyster (C. gigas) + Brown mussel (P.perna) • Tambacu and tambatinga (9%) (hybrids) (22%) 82% • Carps (4%) (≠’s spp.) • Spotted catfish (4%) (Psedoplatystoma spp.) • Others (12%) Inland aquaculture Mariculture Source: IBGE, Ministry of Fisheries and Aquaculture Historical driving forces in the Brazilian aquaculture » Increase in demand of seafood » 2005 = 6,6 kg/habitant/year 2015 = 10,6 kg/habitant/year (+60,6%) » 1 billion US$ seafood imports » Low scale of producers » Most of producers with annual production of less than 5.000 tones » Few producers organizations » Low technological intensity » Innovation gap on native species Aquaculture production in Brazil by category of species (tons) Dourado (Salminus brasiliensis) 38 Peacock -

BMC Evolutionary Biology, 2014, 14
Abe et al. BMC Evolutionary Biology 2014, 14:152 http://www.biomedcentral.com/1471-2148/14/152 RESEARCH ARTICLE Open Access Systematic and historical biogeography of the Bryconidae (Ostariophysi: Characiformes) suggesting a new rearrangement of its genera and an old origin of Mesoamerican ichthyofauna Kelly T Abe, Tatiane C Mariguela, Gleisy S Avelino, Fausto Foresti and Claudio Oliveira* Abstract Background: Recent molecular hypotheses suggest that some traditional suprageneric taxa of Characiformes require revision, as they may not constitute monophyletic groups. This is the case for the Bryconidae. Various studies have proposed that this family (considered a subfamily by some authors) may be composed of different genera. However, until now, no phylogenetic study of all putative genera has been conducted. Results: In the present study, we analyzed 27 species (46 specimens) of all currently recognized genera of the Bryconidae (ingroup) and 208 species representing all other families and most genera of the Characiformes (outgroup). Five genes were sequenced: 16SrRNA, Cytochrome b, recombination activating gene 1 and 2 and myosin heavy chain 6 cardiac muscle. The final matrix contained 4699 bp and was analyzed by maximum likelihood, maximum parsimony and Bayesian analyses. The results show that the Bryconidae, composed of Brycon, Chilobrycon, Henochilus and Salminus, is monophyletic and is the sister group of Gasteropelecidae + Triportheidae. However, the genus Brycon is polyphyletic. Fossil studies suggest that the family originated approximately 47 million years ago (Ma) and that one of the two main lineages persisted only in trans-Andean rivers, including Central American rivers, suggesting a much older origin of Mesoamerican ichthyofauna than previously accepted. -

Genetic Structuring of Salminus Hilarii Valenciennes, 1850 (Teleostei
Neotropical Ichthyology, 13(3): 547-556, 2015 Copyright © 2015 Sociedade Brasileira de Ictiologia DOI: 10.1590/1982-0224-20150015 Genetic structuring of Salminus hilarii Valenciennes, 1850 (Teleostei: Characiformes) in the rio Paraná basin as revealed by microsatellite and mitochondrial DNA markers Juliana Viana da Silva1, Eric M. Hallerman2, Laura Helena Orfão3 and Alexandre Wagner Silva Hilsdorf1 Genetic variation of Salminus hilarii was assessed by screening microsatellite loci and mitochondrial D-loop DNA across four sampling in the upper rio Paraná basin of Brazil. Genetic diversity – measured as mean expected heterozygosity (0.904) and mean number of alleles across populations (13.7) – was reasonably high. Differentiation of microsatellite allele frequencies among populations was shown to be low but significant by AMOVAΦ ST (0.0192), and high by DEST (0.185). D-loop variation was high, with haplotypic diversity of 0.950 and nucleotide diversity of 0.011. Mitochondrial DNA-based estimates for population differentiation were high, with an overall ΦST of 0.173. The results of tests of nuclear and mitochondrial variation yielded no unequivocal inference of historical demographic bottleneck or expansion. Genetic differentiation observed among S. hilarii populations in the rio Grande may be caused by a combination of historical differentiation and recent gene-flow disruption caused by the dams followed by reproduction of isolated spawning assemblages in mid-sized tributaries of the respective reservoirs. We present spatially more intensive sampling of S. hilarii populations across the rio Paraná basin in order to more effectively distinguish between historical and contemporary differentiation. A variabilidade genética de Salminus hilarii foi avaliada por lócus microssatélites e sequências D-Loop do DNA mitocondrial em quatro populações da região da bacia do Alto Paraná. -

The Unique Karyotype of Henochilus Wheatlandii, a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil
The Unique Karyotype of Henochilus wheatlandii,a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil Priscilla C. Silva1*, Udson Santos1, Nata´lia M. Travenzoli1, Jose C. Zanuncio1, Marcelo de B. Cioffi2, Jorge A. Dergam1 1 Laborato´rio de Sistema´tica Molecular - Beagle, Departamento de Biologia Animal, Universidade Federal de Vic¸osa, Vic¸osa, Brazil, 2 Laborato´rio de biodiversidade molecular e citogene´tica, Departamento de Gene´tica e Evoluc¸a˜o, Universidade Federal de Sa˜o Carlos, Sa˜o Carlo, Brazil Abstract Henochilus wheatlandii, the only species of this genus, is critically endangered and was considered extinct for over a century. The rediscovery of this fish in 1996 made it possible to study its phylogenetic relationships with other species in the subfamily Bryconinae. The aim of this study was to characterise the karyotype of H. wheatlandii. Standard staining, C- positive heterochromatin and nucleolar organiser region (NOR) banding, chromomycin A3 staining, and fluorescent in situ hybridisation (FISH) using 5S rDNA and 18S rDNA probes were conducted on nineteen specimens collected in the Santo Antonio River, a sub-basin of the Doce River in Ferros municipality, Minas Gerais State, Brazil. Henochilus wheatlandii shared the same diploid number and chromosome morphology as other species of Bryconinae. However, its heterochromatin distribution patterns, NOR localisation, and FISH patterns revealed a cytogenetic profile unique among Neotropical Bryconinae, emphasizing the evolutionary uniqueness of this threatened species. Citation: Silva PC, Santos U, Travenzoli NM, Zanuncio JC, Cioffi MdB, et al. (2012) The Unique Karyotype of Henochilus wheatlandii, a Critically Endangered Fish Living in a Fast-Developing Region in Minas Gerais State, Brazil. -
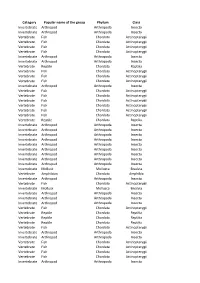
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Conservation Status and Bio-Ecology of Brycon Orbignyanus
Neotropical Ichthyology, 17(3): e190030, 2019 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20190030 Published online: 24 October 2019 (ISSN 1982-0224) Copyright © 2019 Sociedade Brasileira de Ictiologia Printed: 30 September 2019 (ISSN 1679-6225) Original article Conservation status and bio-ecology of Brycon orbignyanus (Characiformes: Bryconidae), an endemic fish species from the Paraná River basin (Brazil) threatened with extinction Lívia Helena Tonella1, Rosa Maria Dias1,2, Oscar Barroso Vitorino Junior1, Rosemara Fugi1,2 and Angelo Antonio Agostinho1,2 Brycon orbignyanus is an endemic species from La Plata basin whose stocks have been presenting significant reductions throughout the Paraná River. Brycon orbignyanus is categorized as an endangered species. This study evaluated aspects of the bio-ecology of this species that may be related to this threat, highlighting its distribution, abundance, and diet as well as the corresponding relationships between its recruitment and flood regimes. Data were obtained from different parts of the upper Paraná River (stretches free and regulated by dams) from 1986 to 2010 with more detailed data collected from the free remnant of this basin. The results indicate that no records for species exist at more than half of the sampling points located in dam-regulated sections of the Paraná River, whereas specimens were collected from 75% sites in the free plain remnant. We observed a remarkable effect of the hydrological regime on recruitment as well as distinct food demands during ontogenetic development, with adults almost exclusively consuming fruits and seeds, revealing that these individuals are supported by riparian vegetation. Thus, it is concluded that changes in the natural flood regime as well as riparian vegetation removal threaten B. -

Parasitizing Gills of Salminus Hilarii from a Neotropical Reservoir, Brazil Revista Brasileira De Parasitologia Veterinária, Vol
Revista Brasileira de Parasitologia Veterinária ISSN: 0103-846X [email protected] Colégio Brasileiro de Parasitologia Veterinária Brasil Brandão, Heleno; Hideki Yamada, Fábio; de Melo Toledo, Gislayne; Carvalho, Edmir Daniel; da Silva, Reinaldo José Monogeneans (Dactylogyridae) parasitizing gills of Salminus hilarii from a Neotropical reservoir, Brazil Revista Brasileira de Parasitologia Veterinária, vol. 22, núm. 4, octubre-diciembre, 2013, pp. 579-587 Colégio Brasileiro de Parasitologia Veterinária Jaboticabal, Brasil Available in: http://www.redalyc.org/articulo.oa?id=397841490019 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Original Article Rev. Bras. Parasitol. Vet., Jaboticabal, v. 22, n. 4, p. 579-587, out.-dez. 2013 ISSN 0103-846X (impresso) / ISSN 1984-2961 (eletrônico) Monogeneans (Dactylogyridae) parasitizing gills of Salminus hilarii from a Neotropical reservoir, Brazil Monogenéticos (Dactylogyridae) parasitando brânquias de Salminus hilarii de uma represa Neotropical, Brasil Heleno Brandão1*; Fábio Hideki Yamada1; Gislayne de Melo Toledo1; Edmir Daniel Carvalho2; Reinaldo José da Silva1 1Laboratório de Parasitologia de Animais Silvestres – LAPAS, Departamento de Parasitologia, Instituto de Biociências, UNESP – Universidade Estadual Paulista, Botucatu, SP, Brasil 2Laboratório de Biologia e Ecologia de Peixes, Departamento de Morfologia, Instituto de Biociências, UNESP – Universidade Estadual Paulista, Botucatu, SP, Brasil †Edmir Daniel de Carvalho (in memory) Received August 13, 2013 Accepted November 1, 2013 Abstract With the aim of creating an inventory of the metazoan gill parasites of Salminus hilarii in the Taquari River, state of São Paulo, Brazil, five species of monogeneans (Anacanthorus contortus, A. -

Stress Evaluation in Dourado Females (Salminus Brasiliensis) Submitted to Two Different Methods of Induced Spawning
Vol. 11(5), pp. 97-103, June 2019 DOI: 10.5897/IJFA2019.0726 Article Number: CA72B1261205 ISSN 2006-9839 Copyright ©2019 International Journal of Fisheries and Author(s) retain the copyright of this article http://www.academicjournals.org/IJFA Aquaculture Full Length Research Paper Stress evaluation in dourado females (Salminus brasiliensis) submitted to two different methods of induced spawning M. A. Zanoni, S. Carvalho, F. G. Costa and F. R. F. Seiva Department of Biology, Center for Biological Sciences, State University of North Paraná (UENP), Rodovia BR-369 Km 54, Vila Maria, CP 261 - CEP 86360-000 Bandeirantes – Paraná, Brasil. Received 13 February, 2019; Accepted 23 May, 2019 The goals of this study were to evaluate dourado female egg viability submitted to stress condition caused by two different reproductive induction methods, extruded and semi-natural. Sixteen females randomly chosen were induced with pituitary extract and allocated in two groups; the first, in which oocytes were manually extruded and the second group, females were allowed to naturally spaw. Blood samples were collected for glucose, cortisol and hematocrit determinations as well as for red blood cells and white blood cells smears evaluations. A control group had its blood collected before hormonal induction. Fertilization rate was 40.6 and 91.7% for extruded and semi-natural methods, respectively, and the survival rate was higher in semi-natural groups. Both experimental groups showed higher glucose and cortisol levels compared to control group. The results for hematocrit, hemoglobin concentration and erythrocytes numbers did not differ among groups. Extruded and semi- natural procedures elevated monocytes percentage. -

Brycon Orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae): Basis for Management Programs
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Portal de Revistas do ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) 18 Número misto: Conservação de Peixes Continentais e Manejo de Unidades de Conservação Conservation Status of the “Piracanjuba” Brycon orbignyanus (Valenciennes, 1850) (Characiformes, Bryconidae): Basis for Management Programs Daniela José de Oliveira¹, Fernando Yuldi Ashikaga¹, Fausto Foresti¹ & José Augusto Senhorini² Recebido em 28/05/2015 – Aceito em 07/04/2016 RESUMO – A exploração excessiva dos recursos de água doce tem causado muitos impactos sobre as populações naturais, especialmente em peixes. Diferentes fatores relacionados com a poluição, sobrepesca, introdução de espécies exóticas, construção de barragens e destruição de vegetação ripárias podem determinar mudanças nas populações atuais e interferir em seu equilíbrio. Neste contexto, Brycon orbignyanus, popularmente conhecido como “piracanjuba”, é uma espécie de peixe ameaçada de extinção pela pesca excessiva e degradação do meio ambiente. A qualidade da carne e do seu comportamento agressivo na prática da pesca desportiva e profissional, ocorrendo concomitantemente às mudanças contínuas no habitat, resultaram no esgotamento rápido dos estoques naturais e determinaram sua entrada na lista de espécies de peixes brasileiros ameaçados de extinção. A espécie tem distribuição ao longo da bacia do Rio da Prata em áreas altamente impactadas pela ocupação demográfica, construção de barragens e atividades agrícolas em áreas marginais. Populações nativas desta espécie ocupam regiões menos afetadas, sendo atualmente restrita a poucos rios distantes de centros urbanos e pequenos afluentes onde as condições ambientais permanecem preservadas. Com o objetivo de verificar o estado de conservação das populações de B. -

Serrasalmus Humeralis) Ecological Risk Screening Summary
Pirambeba (Serrasalmus humeralis) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2012 Revised, July 2018 Web Version, 9/11/2020 Organism Type: Fish Overall Risk Assessment Category: Uncertain Image: Francis de Laporte de Castelnau. Public domain. Available: http://eol.org/pages/211487/overview. (July 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “South America: Amazon River basin.” 1 Froese and Pauly (2018) report that Serrasalmus humeralis is native to Bolivia, Brazil, and Peru, and that records from French Guiana are a misidentification. Maldonado-Ocampo et al. (2008) report S. humeralis from the Amazon basin in Colombia. Status in the United States Specimens from the breeding population described in the quotations below were later re- examined and identified as Serrasalmus rhombeus (see Remarks). From Shafland and Foote (1979): “An isolated reproducing population of the pirambeba, Serrasalmus humeralis, was recently discovered at a Dade County, Florida tourist attraction. Twenty to 25 unidentified Serrasalmus sp. had been stocked into the ‘piranha pool’ in 1963 or 1964. The only fish kill noted during the 13 to 14 yr [sic] history of this population occurred during a severe drought in 1971 when the pool dried into a small mud-puddle. A few S. humeralis survived this drought and continued reproducing.” From Froese and Pauly (2018): “[…] breeding population was eradicated deliberately in 1981 in Florida.” This species is in trade in the United States. For example: From AquaScapeOnline (2018): “Humeralis Piranha 5" (Serrasalmus Humeralis [sic]) […] New Piranha. Seldom seen available for sale. […] Our Price: $225.00” Possession or importation of fish of the genus Serrasalmus, or fish known as “piranha” in general, is banned or regulated in many States.