Import of Live Goats and Sheep from Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Partnership Fact Sheet
PARTNERSHIP FACT SHEET PORTMORE, JAMAICA + TOWNSVILLE, AUSTRALIA LOCATED IN THE ATLANTIC HURRICANE BELT, Portmore, Jamaica is extremely susceptible to hurricanes that RESULTS can cause severe flooding and widespread infrastructure damage. Portmore is a low-lying area on the southern coast of Jamaica. 1 Originally a predominantly agricultural area, the city transformed into a large residential community in the 1950s and became home Based off of a collective social learning for thousands of residents who worked in Kingston. Since then, workshop model from Townsville, the the population of Portmore has grown extremely rapidly, leading partnership hosted a workshop for 46 key it to become the largest residential area in the Caribbean. stakeholders from local government, civil society, and the national government in One of the greatest climate related risks to Portmore is the Portmore to prioritize climate actions that will potential impacts from tropical storms, storm surges and sea feed into Portmore’s Climate Action Plan. level rise. The coastal location of the city also renders it highly susceptible to incremental changes in sea levels and the potential 2 for inundation that will only worsen with future seal level rise. Portmore adopted climate education initiatives from Townsville that will work with students Recognizing that the city’s flood risk is increasing with the threat from elementary to high school on the of climate change, Portmore applied to be part of the CityLinks creation of sensors to monitor indoor energy partnership in the hopes of receiving technical assistance to better consumption and indoor temperatures. plan for future climate impacts. 3 After seeing the impacts white roofs had PARTNERING ON SHARED CLIMATE CHALLENGES in Townsville, Portmore is considering the Although, the distance between Townsville and Portmore design of municipal pilot projects that would couldn’t be greater, local government structure and shared encourage white roofs. -

The Secret History of Australia's Nuclear Ambitions
Jim Walsh SURPRISE DOWN UNDER: THE SECRET HISTORY OF AUSTRALIAS NUCLEAR AMBITIONS by Jim Walsh Jim Walsh is a visiting scholar at the Center for Global Security Research at Lawrence Livermore National Laboratory. He is also a Ph.D. candidate in the Political Science program at MIT, where he is completing a dissertation analyzing comparative nuclear decisionmaking in Australia, the Middle East, and Europe. ustralia is widely considered tactical nuclear weapons. In 1961, of state behavior and the kinds of Ato be a world leader in ef- Australia proposed a secret agree- policies that are most likely to retard forts to halt and reverse the ment for the transfer of British the spread of nuclear weapons? 1 spread of nuclear weapons. The nuclear weapons, and, throughout This article attempts to answer Australian government created the the 1960s, Australia took actions in- some of these questions by examin- Canberra Commission, which called tended to keep its nuclear options ing two phases in Australian nuclear for the progressive abolition of open. It was not until 1973, when history: 1) the attempted procure- nuclear weapons. It led the fight at Australia ratified the NPT, that the ment phase (1956-1963); and 2) the the U.N. General Assembly to save country finally renounced the acqui- indigenous capability phase (1964- the Comprehensive Test Ban Treaty sition of nuclear weapons. 1972). The historical reconstruction (CTBT), and the year before, played Over the course of four decades, of these events is made possible, in a major role in efforts to extend the Australia has gone from a country part, by newly released materials Treaty on the Non-Proliferation of that once sought nuclear weapons to from the Australian National Archive Nuclear Weapons (NPT) indefi- one that now supports their abolition. -

UF in North Queensland, Australia: Sustaining Humans and the Environment Summer A, 2021 Itinerary Highlights
UF in North Queensland, Australia: Sustaining Humans and the Environment Summer A, 2021 Itinerary Highlights Day 1 – 6 Magnetic Island amazing 305 meters into the Stony Creek Gorge and learn about the management plan. Bungalow Bay Koala Village During your stay at Bungalow Bay Koala Village you will learn about their role in conservation on the island. You will receive lectures from their rangers, visit their wildlife sanctuary, and hike through diverse habitats that are home to over 75 species of reptiles, mammals, and birds. Australian Wildlife Conservancy (AWC) AWC's reserves cover more than 7.4 million acres, including the critical conservation of Mt Zero and Taravale. Spend the day working on a range of service-learning activities, such as bush fire control methods of clearing brush, or business assessment and monitoring. Koala population study and beach scrub project Day 10 – 12 Mission Beach to Atherton You will participate in a field-based study surveying the local koala population while enjoying them in the wild, and Aboriginal cultural experience a project on beach scrub, learning about Conservation Learn about Nywaigi Aboriginal culture and hear the Action Planning, how to use camera traps, and conducting stories of their ancestors who were exhibited as cannibals fauna surveys. and savages in nineteenth century circuses in Europe and the US. Try your hand at traditional activities such as throwing boomerangs and spears and take part in a Day 7 – 9 Hidden Valley service-learning project on the Mungalla wetlands. Hidden Valley Cabins ecotourism Stay at a family-run ecotourism business with hosts that are passionate about the natural environment. -

Australia's Political System
Australia’s Political System Australia's Political System Australia's system of government is based on the liberal democratic tradition, which includes religious tolerance and freedom of speech and association. It's institutions and practices reflect British and North American models but are uniquely Australian. The Commonwealth of Australia was created on January 1, 1901 - Federation Day - when six former British colonies - now the six States of Australia - agreed to form a union. The Australian Constitution, which took effect on January 1, 1901, lays down the framework for the Australian system of government. The Constitution The Australian Constitution sets out the rules and responsibilities of government and outlines the powers of its three branches - legislative, executive and judicial. The legislative branch of government contains the parliament - the body with the legislative power to make laws. The executive branch of government administers the laws made by the legislative branch, and the judicial branch of government allows for the establishment of the country's courts of law and the appointment and removal of it judges. The purpose of the courts is to interpret all laws, including the Constitution, making the rule of law supreme. The Constitution can only be changed by referendum. Australia's Constitutional Monarchy Australia is known as a constitutional monarchy. This means it is a country that has a queen or king as its head of state whose powers are limited by a Constitution. Australia's head of state is Queen Elizabeth II. Although she is also Queen of the United Kingdom, the two positions now are quite separate, both in law and constitutional practice. -

Consolidated Practice Directions
CONSOLIDATED PRACTICE DIRECTIONS THE SUPREME COURT OF WESTERN AUSTRALIA 2009 (as updated on 7 October 2021) Unless otherwise indicated, references to the Rules are references to the Rules of the Supreme Court 1971 (WA); and Orders (O) and rules (r) in this document refer to the orders and rules of the Rules of the Supreme Court 1971 (WA). These can be accessed at the Parliamentary Counsel’s Office website at: www.legislation.wa.gov.au Table of Amendments 2012 - 2014 Date Practice Direction (PD) Number Replacement Pages 02/04/2012 Complete Reissue of the Supreme Court's All Consolidated Practice Directions further to a formatting update of the whole document. 27/7/2012 Insertion of PD 9.13 Interpreting and language 269 - 286 Services Guidelines; 9.13.1 Protocol for the Use of Interpreters; and 9.13.2 Interpreter Booking Request Form. 13/8/2012 Insertion of PD 5.8 Case Management. 159 - 168 Further to insertion of PD 9.13 and PD 5.8, the vi - viii & following pages to be reprinted to ensure 159 onwards correct pagination. 17/9/2012 Insertion of new PD 10.5 Practicing Solely as a 305 - 306 & viii Barrister. 21/11/2012 Insertion of new PD 9.1.4 Notice to 197 - 200 Non-Applying Executors. Further to insertion of PD 9.1.4, the following vii - viii & pages to be reprinted to ensure correct 197 onwards pagination. 12/3/2013 Insertion of new allowances in PD 4.7.1.1 - 119 - 121 Schedule of Standard Costs Orders for Interlocutory Applications. 13/3/2013 Insertion of No 18 into PD 4..4.1.1 - Checklist 98 for Entry for Trial. -

CASE STUDY: QUEENSLAND, AUSTRALIA by Judith Sebba, University of Sussex Graham Maxwell, Queensland Studies Authority NOT for CI
WHAT WORKS IN INNOVATION IN EDUCATION CASE STUDY: QUEENSLAND, AUSTRALIA By Judith Sebba, University of Sussex Graham Maxwell, Queensland Studies Authority NOT FOR CITATION BACKGROUND: THE CONTEXT IN THE STATE OF QUEENSLAND, AUSTRALIA Australia is a federation of six states and two territories (also referred to as the Commonwealth of Australia). Under the Australian Constitution, education is a state/territory responsibility and this autonomy is strongly defended on the basis of the need for responsiveness to geographical size and population dispersion, different histories and contexts, and regional needs and circumstances. Most public expenditure on education is sourced from direct or indirect taxation collected at country level and distributed through the states. Schools are government (public/state) or non-government with the latter made up of Catholic and Independent sectors. Approximately one-third of all school students are enrolled in non-government schools. Non-government schools are supported through state and federal government funding. Government school funding is mainly a state/territory matter though some funds also flow from the federal government. Most non-government schools also charge fees. Indigenous students comprise 3.4 per cent of all Australian school students. The percentage of Indigenous students enrolled in Queensland (5.3 per cent) is higher than the national average because of higher concentrations of Indigenous peoples in the north, especially Cape York and the Torres Strait Islands. Most Indigenous students in Queensland, (88 per cent) are enrolled in government schools. Currently, there is compulsory schooling in Queensland for 6-15 year olds (school years 1-10) with the two years of post-compulsory schooling for 16-17 year olds (school years 11-12). -
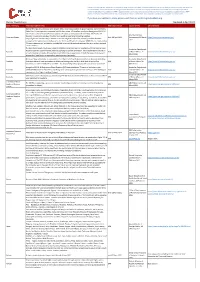
Border Restrictions Updated 6 April 2021
Please note, although we endeavour to provide you with the most up to date information derived from various third parties an d sources, we cannot be held accountable for any inaccuracies or changes to this information. Inclusion of company information in this matrix does no t imply any business relationship between the supplier and WFP / Logistics Cluster, and is used solely as a determinant of services, and capacities. Logistics Cluster /WFP maintain complete impartiality and are not in a position to endorse, comment on any company's suitability as a reputable serv ice provider. If you have any updates to share, please email them to: [email protected] Border Restrictions Updated 6 April 2021 State / Territory Restrictions (Other Info) Restriction Period Source of Info URL / Remarks State of Emergency is extended until 18 April 2021. Color-coded system to guide response. Current level is Code Blue. All entry permits suspended until further notice. All travellers must provide negative COVID-19 test results within 72 hours before arrival and are subject to full quarantine of 14 days. Moreover, the American Samoa traveller is required to disclose if he/she had a positive result prior to testing negative. American Samoa Until 18 April 2021 Government, 19 March https://www.americansamoa.gov/ Cargo flights into the Territory to deliver or retrieve cargo or mail will be allowed, provided that each 2021 occupant of the plane must furnish proof to the Director of Health of a negative COVID-19 test results within 72 hours before arrival, and further provided tht no one will disembark withouth the prior written approval of the Governor. -

Western Australia Growth Perspective
Growth Perspective on Western Australia Ricardo Hausmann, Douglas Barrios, Ana Grisanti, Semiray Kasoolu, Tim O'Brien, Eric Protzer, Rushabh Sanghvi, Nikita Taniparti, Jorge Tapia CID Faculty Working Paper No. 393 Completed May 2020 Published April 2021 Copyright 2021 Hausmann, Ricardo; Barrios, Douglas; Grisanti, Ana; Kasoolu, Semiray; O’Brien, Tim; Protzer, Eric; Sanghvi, Rushabh; Taniparti, Nikita; Tapia, Jorge; and the President and Fellows of Harvard College Working Papers Center for International Development at Harvard University GROWTH PERSPECTIVE ON WESTERN AUSTRALIA May 2020 Growth Lab Center for International Development Harvard University Table of Contents 1. Introduction 2 2. Executive Summary 3 3. Economic Growth Trajectory 5 4. Labor Market Imbalances 10 Recent patterns of employment and wage growth 10 Factors that influenced the limited labor supply response 14 5. Pro-Cyclical Fiscal Policy 20 Recent evolution of the fiscal balance 20 Factors that influenced the pro-cyclicality of fiscal policy 25 6. Infrastructure Policy Misalignment 28 Electricity 29 Water 31 Common Themes 33 7. Conclusions 37 8. References 38 1 | Western Australia Growth Perspective 1. Introduction The Government of Western Australia (WA), acting through its Department of Primary Industries and Regional Development (DPIRD), invited the Growth Lab of the Center for International Development at Harvard University to partner with the state to better understand and address constraints to economic diversification through a collaborative applied research project. The project seeks to apply growth diagnostic and economic complexity methodologies to inform policy design in order to accelerate productive transformation, economic diversification, and more inclusive and resilient job creation across Western Australia. As its name implies, this Growth Perspective Report aims to provide a set of perspectives on the process of economic growth in WA that provide insights for policymakers toward improving growth outcomes. -

Australia's System of Government
61 Australia’s system of government Australia is a federation, a constitutional monarchy and a parliamentary democracy. This means that Australia: Has a Queen, who resides in the United Kingdom and is represented in Australia by a Governor-General. Is governed by a ministry headed by the Prime Minister. Has a two-chamber Commonwealth Parliament to make laws. A government, led by the Prime Minister, which must have a majority of seats in the House of Representatives. Has eight State and Territory Parliaments. This model of government is often referred to as the Westminster System, because it derives from the United Kingdom parliament at Westminster. A Federation of States Australia is a federation of six states, each of which was until 1901 a separate British colony. The states – New South Wales, Victoria, Queensland, Western Australia, South Australia and Tasmania - each have their own governments, which in most respects are very similar to those of the federal government. Each state has a Governor, with a Premier as head of government. Each state also has a two-chambered Parliament, except Queensland which has had only one chamber since 1921. There are also two self-governing territories: the Australian Capital Territory and the Northern Territory. The federal government has no power to override the decisions of state governments except in accordance with the federal Constitution, but it can and does exercise that power over territories. A Constitutional Monarchy Australia is an independent nation, but it shares a monarchy with the United Kingdom and many other countries, including Canada and New Zealand. The Queen is the head of the Commonwealth of Australia, but with her powers delegated to the Governor-General by the Constitution. -

Queensland, Australia
Global Education Education Global AUSTRALIA QUEENSLAND, QUEENSLAND, (CGE) for Center HOBART AND WILLIAM SMITH COLLEGES SMITH WILLIAM AND HOBART Eligibility This program is open to all sophomores, juniors, and seniors in good academic and social standing with a minimum GPA of 2.5 It is expected that students will have taken at least one introductory science course with a lab, although preference is given to students with stronger science preparation. A course in Environmental Studies is recommended but not required. Due to the challenging nature of study abroad, student academic and disciplinary records will be carefully screened. Students must also be cleared for participation by their physician for this physically-demanding program. Accommodations Students will be placed in homestays while in Brisbane and will stay in a variety of accommodation types while in the field, including hotels, research centers, and hostels. Excursions A key feature of the program is a rigorous schedule of multi-day excursions to conduct field work at four sites in Australia: 1) North Stradbroke Island is a sand island in Moreton Bay about 2 hours from Brisbane http://www.cms.uq.edu.au/index.html? page=52452&pid=52450; 2) Lamington Heron Island, on the Southern Great Barrier Reef National Park is a subtropical rainforest three hours from Brisbane http://www.lamingtonnationalpark.net.au/ MainMenu.html 3) Heron Island Research Station is situated on the southern Great Barrier Reef http:// www.science.uq.edu.au/facilities/heron-island and 4) Girraween National Park, in the Tablelands area on the border of Queensland and New South Wales, is a drier region with massive granite outcrops, balancing boulders, diverse flora and fauna, and Aborginal origins http://www.nprsr.qld.gov.au/parks/girraween/index.html. -

'Core' Culture Hegemony and Multiculturalism
ARTICLE Copyright © 2006 SAGE Publications (London,Thousand Oaks, CA and New Delhi) 1468-7968 Vol 6(2): 203–230;063753 DOI:10.1177/1468796806063753 http://etn.sagepub.com ‘Core’ culture hegemony and multiculturalism Perceptions of the privileged position of Australians with British backgrounds JAMES FORREST Macquarie University,Australia KEVIN DUNN University of New South Wales,Australia ABSTRACT Tensions between acceptance of policies aimed at creating a multi- cultural society and British (Anglo or Anglo-Celtic) Australians concerned about loss of their privileged position as members of the dominant society have been an important feature of political debate in Australia in recent years. There is, however, a paucity of empirical evidence available to assess the extent of recognition of Anglo privilege in this debate. This study draws on questions about attitudes to multi- cultural values and Anglo privilege from a recent survey of New South Wales and Queensland respondents to address this issue. Principal components analysis of the attitudinal data shows that multiculturalism and privilege are separate, independent dimensions in respondents’ thinking. Cross-tabulations show both polarization of views and ambivalence in attitudes to Anglo privilege, which are in substantial part resolved by consideration of the geography of privilege and linked multicultural values. KEYWORDS Australia ● geography ● multiculturalism ● White (Anglo) privilege INTRODUCTION Tensions surrounding actual or perceived loss of hegemony of previously dominant Anglo cultures are an increasing feature of major immigrant 204 ETHNICITIES 6(2) receiving countries of the English-speaking world. Typically, these arise from concerns about and complex interrelationships among issues of national identity, citizenship, dominant society privilege and the politics of multiculturalism. -

Climate Change and Migration in the Pacific
KEY FINDINGS CLIMATE IMPACTS People in Kiribati, Nauru, and Tuvalu are already experiencing climate Men and women experience migration differently. Women are slightly change impacts: incremental sea level rise, saltwater intrusion, and more likely to migrate for education and men are more likely to migrate drought. For example, most households in all three countries have been for work. impacted by climate change over the past 10 years (94% in Kiribati, 97% in Migration demand is greater than the access to migration opportu- Tuvalu and 74% in Nauru). This motivates some people to search for new nities. Approximately 10,000 people across Kiribati, Nauru, and Tuvalu homes – either to ensure a source of income or to fi nd land on which to live. attempted to migrate between 2005 and 2015 but were unable to do so, Climate change is already impacting migration patterns in Kiribati and primarily due to fi nancial constraints. Tuvalu. Today, 23% of migrants in Kiribati and 8% in Tuvalu named climate change as a reason for migration decisions. Future impacts of climate change on migration Climate change will drastically impact pressures to migrate, particu- International and internal migration history larly in Kiribati and Tuvalu. More than 70% of households in Kiribati and The potential for Pacifi c households to use international migration to Tuvalu, and 35% in Nauru felt that migration would be a likely response if manage the risks of climate stressors is limited by lack of access to in- droughts, sea level rise or fl oods worsened. Many potential migrants will ternational migration opportunities. The international migration opportu- not have the means to migrate.