Living in a Changing World: Effects of Roads and Pinus Monocultures On
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Histomorfología De La Glándula Tiroides Durante La Ontogenia En Pseudis Paradoxa (Anura, Hylidae)
Tesis Doctoral Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae) Cruz, Julio César 2017 Este documento forma parte de las colecciones digitales de la Biblioteca Central Dr. Luis Federico Leloir, disponible en bibliotecadigital.exactas.uba.ar. Su utilización debe ser acompañada por la cita bibliográfica con reconocimiento de la fuente. This document is part of the digital collection of the Central Library Dr. Luis Federico Leloir, available in bibliotecadigital.exactas.uba.ar. It should be used accompanied by the corresponding citation acknowledging the source. Cita tipo APA: Cruz, Julio César. (2017). Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae). Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. https://hdl.handle.net/20.500.12110/tesis_n6259_Cruz Cita tipo Chicago: Cruz, Julio César. "Histomorfología de la glándula tiroides durante la ontogenia en Pseudis paradoxa (Anura, Hylidae)". Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. 2017. https://hdl.handle.net/20.500.12110/tesis_n6259_Cruz Dirección: Biblioteca Central Dr. Luis F. Leloir, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Contacto: bibliotecadigital.exactas.uba.ar Intendente Güiraldes 2160 - C1428EGA - Tel. (++54 +11) 4789-9293 UNIVERSIDAD DE BUENOS AIRES Facultad de Ciencias Exactas y Naturales Departamento de Biodiversidad y Biología Experimental Histomorfología de la glándula tiroides durante la ontogenia -
For Review Only
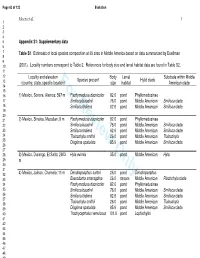
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Community Structure of Parasites of the Tree Frog Scinax Fuscovarius (Anura, Hylidae) from Campo Belo Do Sul, Santa Catarina, Brazil
ISSN Versión impresa 2218-6425 ISSN Versión Electrónica 1995-1043 ORIGINAL ARTICLE /ARTÍCULO ORIGINAL COMMUNITY STRUCTURE OF PARASITES OF THE TREE FROG SCINAX FUSCOVARIUS (ANURA, HYLIDAE) FROM CAMPO BELO DO SUL, SANTA CATARINA, BRAZIL ESTRUCTURA DE LA COMUNIDAD PARASITARIA DE LA RANA ARBORICOLA SCINAX FUSCOVARIUS (ANURA, HYLIDAE) DE CAMPO BELO DO SUL, SANTA CATARINA, BRASIL Viviane Gularte Tavares dos Santos1,2; Márcio Borges-Martins1,3 & Suzana B. Amato1,2 1 Departamento de Zoologia, Programa de Pós-graduação em Biologia Animal, Instituto de Biociências, Universidade Federal do Rio Grande do Sul, Porto Alegre, 91501-970, Rio Grande do Sul, Brasil. 2 Laboratório de Helmintologia; Universidade Federal do Rio Grande do Sul, Porto Alegre, 91501-970, Rio Grande do Sul, Brasil. 3 Laboratório de Herpetologia. Universidade Federal do Rio Grande do Sul, Porto Alegre, 91501-970, Rio Grande do Sul, Brasil. E-mail: [email protected]; [email protected]; [email protected] Neotropical Helminthology, 2016, 10(1), ene-jun: 41-50. ABSTRACT Sixty specimens of Scinax fuscovarius (Lutz, 1925) were collected between May 2009 and October 2011 at Campo Belo do Sul, State of Santa Catarina, Brazil, and necropsied in search of helminth parasites. Only four helminth species were found: Pseudoacanthocephalus sp. Petrochenko, 1958, Cosmocerca brasiliense Travassos, 1925, C. parva Travassos, 1925 and Physaloptera sp. Rudolphi, 1819 (larvae). The genus of the female cosmocercids could not be determined. Only 30% of the anurans were parasitized. Scinax fuscovarius presented low prevalence, infection intensity, and parasite richness. Sex and size of S. fuscovarius individuals did not influence the prevalence, abundance, and species richness of helminth parasites. -

Mannophryne Olmonae) Catherine G
The College of Wooster Libraries Open Works Senior Independent Study Theses 2014 A Not-So-Silent Spring: The mpI acts of Traffic Noise on Call Features of The loB ody Bay Poison Frog (Mannophryne olmonae) Catherine G. Clemmens The College of Wooster, [email protected] Follow this and additional works at: https://openworks.wooster.edu/independentstudy Part of the Other Environmental Sciences Commons Recommended Citation Clemmens, Catherine G., "A Not-So-Silent Spring: The mpI acts of Traffico N ise on Call Features of The loodyB Bay Poison Frog (Mannophryne olmonae)" (2014). Senior Independent Study Theses. Paper 5783. https://openworks.wooster.edu/independentstudy/5783 This Senior Independent Study Thesis Exemplar is brought to you by Open Works, a service of The oC llege of Wooster Libraries. It has been accepted for inclusion in Senior Independent Study Theses by an authorized administrator of Open Works. For more information, please contact [email protected]. © Copyright 2014 Catherine G. Clemmens A NOT-SO-SILENT SPRING: THE IMPACTS OF TRAFFIC NOISE ON CALL FEATURES OF THE BLOODY BAY POISON FROG (MANNOPHRYNE OLMONAE) DEPARTMENT OF BIOLOGY INDEPENDENT STUDY THESIS Catherine Grace Clemmens Adviser: Richard Lehtinen Submitted in Partial Fulfillment of the Requirement for Independent Study Thesis in Biology at the COLLEGE OF WOOSTER 2014 TABLE OF CONTENTS I. ABSTRACT II. INTRODUCTION…………………………………………...............…...........1 a. Behavioral Effects of Anthropogenic Noise……………………….........2 b. Effects of Anthropogenic Noise on Frog Vocalization………………....6 c. Why Should We Care? The Importance of Calling for Frogs..................8 d. Color as a Mode of Communication……………………………….…..11 e. Biology of the Bloody Bay Poison Frog (Mannophryne olmonae)…...13 III. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Anuran Assemblage Changes Along Small-Scale Phytophysiognomies in Natural Brazilian Grasslands
bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.229310; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Anuran assemblage changes along small-scale phytophysiognomies in natural Brazilian grasslands Diego Anderson Dalmolin1*, Volnei Mathies Filho2, Alexandro Marques Tozetti3 1 Laboratório de Metacomunidades, Departamento de Ecologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. 2 Fundação Universidade Federal do Rio Grande, Rio Grande, Rio Grande do Sul, Brasil 3Laboratório de Ecologia de Vertebrados Terrestres, Universidade do Vale do Rio dos Sinos, Avenida Unisinos 950, 93022-000 São Leopoldo, Rio Grande do Sul, Brazil. * Corresponding author: Email: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.07.31.229310; this version posted August 3, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Abstract 2 We studied the species composition of frogs in two phytophysiognomies (grassland and 3 forest) of a Ramsar site in southern Brazil. We aimed to assess the distribution of 4 species on a small spatial scale and dissimilarities in community composition between 5 grassland and forest habitats. The sampling of individuals was carried out through 6 pitfall traps and active search in the areas around the traps. -

Check List 8(1): 102-111, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(1): 102-111, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Frogs and toads of the Pedra Azul–Forno Grande PECIES S Biodiversity Corridor, southeastern Brazil OF Rachel Montesinos 1*, Pedro L.V. Peloso 2, Diogo A. Koski 3, Aline P. Valadares 4 and João Luiz Gasparini 5 ISTS L 1 Universidade Federal Rural do Rio de Janeiro, Instituto de Biologia, Laboratório de Herpetologia, Caixa Postal 74524. CEP 23851-970. Seropédica, RJ, Brazil. 2 Division of Vertebrate Zoology (Herpetology) and Richard Gilder Graduate School, American Museum of Natural History, Central Park West at 79th Street, New York, 10024, NY, USA. Brazil. 43 CentroAssociação Universitário Educacional Vila de Velha Vitória – UVV. (AEV/FAESA), Rua Comissário Instituto José SuperiorDantas de de Melo, Educação. 21, Boa Rodovia Vista. CEP Serafim 29102-770. Derenzi, Vila 3115. Velha, CEP ES, 29048-450. Brazil. Vitória, ES, Vitória, ES, Brazil. *5 CorrespondingUniversidade Federal author: do [email protected] Espírito Santo, Departamento de Ecologia e Oceanografia. Avenida Fernando Ferrari, 514, Goiabeiras. CEP 29075-910. Abstract: We conducted a long-term amphibian survey at the biodiversity corridor Pedra Azul-Forno Grande, in the mountain region of the state of Espírito Santo, Brazil. Sampling was conducted from April 2004 to October 2009 and we registered 43 species. Two species (Dendropsophus ruschii and Megaelosia apuana) are included in the state list of threatened species and Scinax belloni is included in the IUCN/GAA list. We provide color photographs for most species found in the region. -

Systematics of the Dendropsophus Leucophyllatus Species Complex (Anura: Hylidae): Cryptic Diversity and the Description of Two New Species
RESEARCH ARTICLE Systematics of the Dendropsophus leucophyllatus species complex (Anura: Hylidae): Cryptic diversity and the description of two new species Marcel A. Caminer1,2, Borja MilaÂ2, Martin Jansen3, Antoine Fouquet4, Pablo J. Venegas1,5, GermaÂn ChaÂvez5, Stephen C. Lougheed6, Santiago R. Ron1* a1111111111 1 Museo de ZoologõÂa, Escuela de BiologõÂa, Pontificia Universidad CatoÂlica del Ecuador, Quito, Ecuador, 2 National Museum of Natural Sciences, Spanish Research Council (CSIC), Madrid, Spain, 3 Senckenberg a1111111111 Gesellschaft fuÈr Naturforschung, Frankfurt am Main, Germany, 4 CNRS Guyane USR LEEISA, Centre de a1111111111 recherche de Montabo, Cayenne, French Guiana, 5 DivisioÂn de HerpetologõÂa-Centro de OrnitologõÂa y a1111111111 Biodiversidad (CORBIDI), Urb. Huertos de San Antonio, Surco, Lima-PeruÂ, 6 Department of Biology, a1111111111 Queen's University, Kingston, Ontario, Canada * [email protected] OPEN ACCESS Abstract Citation: Caminer MA, Mila B, Jansen M, Fouquet A, Venegas PJ, ChaÂvez G, et al. (2017) Systematics Genetic data in studies of systematics of Amazonian amphibians frequently reveal that pur- of the Dendropsophus leucophyllatus species portedly widespread single species in reality comprise species complexes. This means that complex (Anura: Hylidae): Cryptic diversity and the real species richness may be significantly higher than current estimates. Here we combine description of two new species. PLoS ONE 12(3): genetic, morphological, and bioacoustic data to assess the phylogenetic relationships and e0171785. doi:10.1371/journal.pone.0171785 species boundaries of two Amazonian species of the Dendropsophus leucophyllatus spe- Editor: Stefan LoÈtters, Universitat Trier, GERMANY cies group: D. leucophyllatus and D. triangulum. Our results uncovered the existence of five Received: October 12, 2016 confirmed and four unconfirmed candidate species. -

The International Journal of the Willi Hennig Society
Cladistics VOLUME 35 • NUMBER 5 • OCTOBER 2019 ISSN 0748-3007 Th e International Journal of the Willi Hennig Society wileyonlinelibrary.com/journal/cla Cladistics Cladistics 35 (2019) 469–486 10.1111/cla.12367 A total evidence analysis of the phylogeny of hatchet-faced treefrogs (Anura: Hylidae: Sphaenorhynchus) Katyuscia Araujo-Vieiraa, Boris L. Blottoa,b, Ulisses Caramaschic, Celio F. B. Haddadd, Julian Faivovicha,e,* and Taran Grantb,* aDivision Herpetologıa, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”-CONICET, Angel Gallardo 470, Buenos Aires, C1405DJR, Argentina; bDepartamento de Zoologia, Instituto de Biociencias,^ Universidade de Sao~ Paulo, Sao~ Paulo, Sao~ Paulo, 05508-090, Brazil; cDepartamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, Sao~ Cristov ao,~ Rio de Janeiro, Rio de Janeiro, 20940-040, Brazil; dDepartamento de Zoologia and Centro de Aquicultura (CAUNESP), Instituto de Biociencias,^ Universidade Estadual Paulista, Avenida 24A, 1515, Bela Vista, Rio Claro, Sao~ Paulo, 13506–900, Brazil; eDepartamento de Biodiversidad y Biologıa Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina Accepted 14 November 2018 Abstract The Neotropical hylid genus Sphaenorhynchus includes 15 species of small, greenish treefrogs widespread in the Amazon and Orinoco basins, and in the Atlantic Forest of Brazil. Although some studies have addressed the phylogenetic relationships of the genus with other hylids using a few exemplar species, its internal relationships remain poorly understood. In order to test its monophyly and the relationships among its species, we performed a total evidence phylogenetic analysis of sequences of three mitochondrial and three nuclear genes, and 193 phenotypic characters from all species of Sphaenorhynchus. -

Diet Composition, Body Condition and Sexual Size Dimorphism of the Common African
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.25.428067; this version posted January 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Diet composition, body condition and sexual size dimorphism of the common African 2 toad (Amietophrynus regularis) in urban and agricultural landscape 3 Benjamin Yeboah Ofori*, John Bosu Mensah, Roger Sigismund Anderson and Daniel Korley 4 Attuquayefio 5 Department of Animal Biology and Conservation Science, University of Ghana, Legon 6 *Corresponding author; Email: [email protected] 7 8 9 Abstract 10 Land use and land cover change (LULCC) are major drivers of global biodiversity loss. The 11 conversion of natural habitats into human-modified landscapes poses novel and multifaceted 12 environmental stressors to organisms, influencing their ecology, physiology, life history and 13 fitness. Although the effects of LULCC have been studied extensively at the community level, 14 there is scant information about its effect on population and individual characteristics. We 15 assessed the diet composition, body condition, and sexual size dimorphism of the common 16 African toad (Amietophrynus regularis) in urban and agricultural landscape. Diet composition 17 was evaluated using gut content analysis, while body condition was measured using residual 18 mass index. Overall, 935 prey items comprising six classes, at least 18 orders and 31 families 19 were obtained from toads. This broad dietary niche suggested that Amietophrynus regularis is a 20 generalist predator. -

Redalyc.Hemoparasites of the Genus Trypanosoma (Kinetoplastida
Anais da Academia Brasileira de Ciências ISSN: 0001-3765 [email protected] Academia Brasileira de Ciências Brasil LEAL, DENISE D.M.; O'DWYER, LUCIA H.; RIBEIRO, VITOR C.; SILVA, REINALDO J.; FERREIRA, VANDA L.; RODRIGUES, ROZANGELA B. Hemoparasites of the genus Trypanosoma (Kinetoplastida: Trypanosomatidae) and hemogregarines in Anurans of the São Paulo and Mato Grosso do Sul States - Brazil Anais da Academia Brasileira de Ciências, vol. 81, núm. 2, junio, 2009, pp. 199-206 Academia Brasileira de Ciências Rio de Janeiro, Brasil Available in: http://www.redalyc.org/articulo.oa?id=32713477006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative “main” — 2009/5/4 — 11:00 — page 199 — #1 Anais da Academia Brasileira de Ciências (2009) 81(2): 199-206 (Annals of the Brazilian Academy of Sciences) ISSN 0001-3765 www.scielo.br/aabc Hemoparasites of the genus Trypanosoma (Kinetoplastida: Trypanosomatidae) and hemogregarines in Anurans of the São Paulo and Mato Grosso do Sul States – Brazil DENISE D.M. LEAL1, LUCIA H. O’DWYER1, VITOR C. RIBEIRO2, REINALDO J. SILVA1, VANDA L. FERREIRA3 and ROZANGELA B. RODRIGUES3 1Departamento de Parasitologia, Instituto de Biociências, Unesp, Distrito Rubião Júnior s/n 18618-000 Botucatu, SP, Brasil 2Instituto de Biotecnologia Aplicada a Agricultura, UFV, Campus Universitário, Avenida Peter Henry Rolfs s/n 36570-000 Viçosa, MG, Brasil 3Departamento de Biologia-Ecologia, Centro de Ciências Biológicas e da Saúde (CCBS), UFMS, Cidade Universitária 79070-900 Campo Grande, MS, Brasil Manuscript received on March 11, 2008; accepted for publication on October 8, 2008; presented by LUIZ R. -

Biodiversity of the Pantanal: Response to Seasonal Flooding Regime and To
Biodiversity of the Pantanal: response to seasonal flooding regime and to environmental degradation Alho, CJR.* Pós-graduação em Meio Ambiente e Desenvolvimento Regional, Universidade Para o Desenvolvimento do Estado e da Região do Pantanal – UNIDERP, Rua Ceará, 333, CEP 79003-010, Campo Grande, MS, Brazil *e-mail: [email protected] Received December 27, 2007 – Accepted December 27, 2007 – Distributed November 30, 2008 (With 1 figure) Abstract Seasonal flooding is the most important ecological phenomenon in the Pantanal. Every year many parts of the biome change from terrestrial into aquatic habitats and vice-versa. The degree of inundation creates a range of major habi- tats. Flooding occupies about 80% of the whole Pantanal. In contrast, during the dry season, most of the flooded areas stay dry, when the water returns to the river beds or evaporates. The Pantanal is a large continental savanna wetland (147,574 km2 in Brazil), touching Bolivia to the north and Paraguay to the south. The maze of fluctuating water levels, nutrients, and biota forms a dynamic ecosystem. The vegetation comprises 1,863 phanerogam plant species listed for the floodplain and 3,400 for the whole basin and 250 species of aquatic plants. The complex vegetation cover and sea- sonal productivity support a diverse and abundant fauna within the floodplain: 263 species of fish, 41 of amphibians, 113 of reptiles (177 for the basin), 463 of birds and 132 mammal species. Many endangered species occur, including jaguar (Panthera onca Linnaeus, 1758). Waterfowl are exceptionally