Pericardial Mesoderm Generates a Population of Coronary Smooth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Genetic and Flow Anomalies in Congenital Heart Disease
Published online: 2021-05-10 AIMS Genetics, 3(3): 157-166. DOI: 10.3934/genet.2016.3.157 Received: 01 July 2016 Accepted: 16 August 2016 Published: 23 August 2016 http://www.aimspress.com/journal/Genetics Review Genetic and flow anomalies in congenital heart disease Sandra Rugonyi* Department of Biomedical Engineering, Oregon Health & Science University, 3303 SW Bond Ave. M/C CH13B, Portland, OR 97239, USA * Correspondence: Email: [email protected]; Tel: +1-503-418-9310; Fax: +1-503-418-9311. Abstract: Congenital heart defects are the most common malformations in humans, affecting approximately 1% of newborn babies. While genetic causes of congenital heart disease have been studied, only less than 20% of human cases are clearly linked to genetic anomalies. The cause for the majority of the cases remains unknown. Heart formation is a finely orchestrated developmental process and slight disruptions of it can lead to severe malformations. Dysregulation of developmental processes leading to heart malformations are caused by genetic anomalies but also environmental factors including blood flow. Intra-cardiac blood flow dynamics plays a significant role regulating heart development and perturbations of blood flow lead to congenital heart defects in animal models. Defects that result from hemodynamic alterations recapitulate those observed in human babies, even those due to genetic anomalies and toxic teratogen exposure. Because important cardiac developmental events, such as valve formation and septation, occur under blood flow conditions while the heart is pumping, blood flow regulation of cardiac formation might be a critical factor determining cardiac phenotype. The contribution of flow to cardiac phenotype, however, is frequently ignored. -

Prenatal Development Timeline
Prenatal Development Timeline Nervous Cardiovascular Muscular Early Events Special Senses Respiratory Skeletal Growth Parameters Blood & Immune Gastrointestinal Endocrine General Skin/Integument Renal/Urinary Reproductive Movement Unit 1: The First Week Day 0 — — Embryonic period begins Fertilization resulting in zygote formation Day 1 — — Embryo is spherically shaped with 12 to 16 cells Day 1 - Day 1 — — Fertilization - development begins with a single-cell embryo!!! Day 2 — — Early pregnancy factor (EPF) Activation of the genome Zygote divides into two blastomeres (24 – 30 hours from start of fertilization) Day 4 — — Embryonic disc Free floating blastocyst Hypoblast & epiblast Inner cell mass See where the back and chest will be Day 5 — — Hatching blastocyst Day 6 — — Embryo attaches to wall of uterus 1 week — — Chorion Placenta begins to form Unit 2: 1 to 2 Weeks 1 week, 1 day — — Amnioblasts present; amnion and amniotic cavity formation begins Positive pregnancy test 1 week, 2 days — — Cells in womb engorged with nutrients 1 week, 4 days — — Longitudinal axis 1 week, 5 days — — Implantation complete Yolk sac 1 week, 6 days — — Primordial blood vessels Amnion with single cell layer Chorionic villi 2 weeks — — Yolk sac Yolk sac Unit 3: 2 to 3 Weeks 2 weeks, 1 day — — 3 germ layers Rostral-caudal orientation 2 weeks, 2 days — — Erythroblasts in yolk sac Three types of blood-forming cells in yolk sac Amnion with two cell layers Secondary villi www.ehd.org 1 of 10 2 weeks, 4 days — — Foregut, midgut, and hindgut Brain is first organ to -
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

The Functional Anatomy of the Heart. Development of the Heart, Anomalies
The functional anatomy of the heart. Development of the heart, anomalies Anatomy and Clinical Anatomy Department Anastasia Bendelic Plan: Cardiovascular system – general information Heart – functional anatomy Development of the heart Abnormalities of the heart Examination in a living person Cardiovascular system Cardiovascular system (also known as vascular system, or circulatory system) consists of: 1. heart; 2. blood vessels (arteries, veins, capillaries); 3. lymphatic vessels. Blood vessels Arteries are blood vessels that carry blood away from the heart. Veins carry blood back towards the heart. Capillaries are tiny blood vessels, that connect arteries to veins. Lymphatic vessels: lymphatic capillaries; lymphatic vessels (superficial and deep lymph vessels); lymphatic trunks (jugular, subclavian, bronchomediastinal, lumbar, intestinal trunks); lymphatic ducts (thoracic duct and right lymphatic duct). Lymphatic vessels Microcirculation Microcirculatory bed comprises 7 components: 1. arterioles; 2. precapillaries or precapillary arterioles; 3. capillaries; 4. postcapillaries or postcapillary venules; 5. venules; 6. lymphatic capillaries; 7. interstitial component. Microcirculation The heart Heart is shaped as a pyramid with: an apex (directed downward, forward and to the left); a base (facing upward, backward and to the right). There are four surfaces of the heart: sternocostal (anterior) surface; diaphragmatic (inferior) surface; right pulmonary surface; left pulmonary surface. External surface of the heart The heart The heart has four chambers: right and left atria; right and left ventricles. Externally, the atria are demarcated from the ventricles by coronary sulcus (L. sulcus coronarius). The right and left ventricles are demarcated from each other by anterior and posterior interventricular sulci (L. sulci interventriculares anterior et posterior). Chambers of the heart The atria The atria are thin-walled chambers, that receive blood from the veins and pump it into the ventricles. -
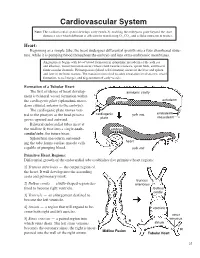
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos
Review Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos Jörg Männer 1,*,† and Talat Mesud Yelbuz 2,† 1 Group Cardio‐Embryology, Institute of Anatomy and Embryology UMG, Georg‐August‐University Goettingen, D‐37075 Goettingen, Germany; [email protected] 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh 11426, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +49‐551‐39‐7032 † This work is dedicated to the memory of our academic mentors Gerd Steding (1936–2011) and Armin Wessel (1946–2011). Received: 29 January 2019; Accepted: 21 February 2019; Published: 27 February 2019 Abstract: The early embryonic heart is a multi‐layered tube consisting of (1) an outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular matrix layer interposed between the myocardium and endocardium, called “cardiac jelly” (CJ). During the past decades, research on CJ has mainly focused on its molecular and cellular biological aspects. This review focuses on the morphological and biomechanical aspects of CJ. Special attention is given to (1) the spatial distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced heart looping stages, which leads to the formation of ventricular trabeculations and endocardial cushions. CJ acts as a hydraulic skeleton, displaying striking structural and functional similarities with the mesoglea of jellyfish. CJ not only represents a filler substance, facilitating end‐systolic occlusion of the embryonic heart lumen. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

Conserved Signaling Mechanisms in Drosophila Heart Development
DEVELOPMENTAL DYNAMICS 246:641–656, 2017 DOI: 10.1002/DVDY.24530 REVIEWS Conserved Signaling Mechanisms in Drosophila Heart Development a Shaad M. Ahmad 1,2* 1Department of Biology, Indiana State University, Terre Haute, Indiana 2The Center for Genomic Advocacy, Indiana State University, Terre Haute, Indiana Abstract: Signal transduction through multiple distinct pathways regulates and orchestrates the numerous biological pro- cesses comprising heart development. This review outlines the roles of the FGFR, EGFR, Wnt, BMP, Notch, Hedgehog, Slit/ Robo, and other signaling pathways during four sequential phases of Drosophila cardiogenesis—mesoderm migration, cardiac mesoderm establishment, differentiation of the cardiac mesoderm into distinct cardiac cell types, and morphogenesis of the heart and its lumen based on the proper positioning and cell shape changes of these differentiated cardiac cells—and illus- trates how these same cardiogenic roles are conserved in vertebrates. Mechanisms bringing about the regulation and combi- natorial integration of these diverse signaling pathways in Drosophila are also described. This synopsis of our present state of knowledge of conserved signaling pathways in Drosophila cardiogenesis and the means by which it was acquired should facili- tate our understanding of and investigations into related processes in vertebrates. Developmental Dynamics 246:641–656, 2017. VC 2017 Wiley Periodicals, Inc. Key words: heart development; Drosophila cardiogenesis; cardiac mesoderm specification; cardiac morphogenesis; -

Functional Morphology of the Cardiac Jelly in the Tubular
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 30 January 2019 doi:10.20944/preprints201901.0312.v1 Peer-reviewed version available at J. Cardiovasc. Dev. Dis. 2019, 6, 12; doi:10.3390/jcdd6010012 1 Review 2 Functional Morphology of the Cardiac Jelly in the 3 Tubular Heart of Vertebrate Embryos 4 Jörg Männer 1,* and Talat Mesud Yelbuz 2 5 1 Group Cardio-Embryology, Institute of Anatomy and Embryology UMG, Georg-August-University 6 Goettingen, D-37075 Goettingen, Germany; [email protected] 7 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King 8 Abdulaziz Medical City, Ministry of National Guard Health Affairs; Riyadh, Kingdom of Saudi Arabia; 9 [email protected] 10 * Correspondence: [email protected]; Tel.: +49-551-39-7032 11 12 Abstract: The early embryonic heart is a multi-layered tube consisting of (1) an 13 outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular 14 matrix layer interposed between myocardium and endocardium, called “cardiac 15 jelly” (CJ). During the past decades, research on CJ has mainly focused on its 16 molecular and cell biological aspects. This review focuses on the morphological 17 and biomechanical aspects of CJ. Special attention is given to (1) the spatial 18 distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ 19 during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced 20 heart looping stages, which leads to the formation of ventricular trabeculations 21 and endocardial cushions. CJ acts as a hydraulic skeleton displaying striking 22 structural and functional similarities with the mesoglea of jellyfish. -

6. Heart and Circulatory System I
6. HEART AND CIRCULATORY SYSTEM I Dr. Taube P. Rothman P&S 12-520 [email protected] 212-305-7930 RECOMMENDED READING: Larsen Human Embryology, 3rd Edition, pp. 195-199; 157-169 top left; 172-174; bottom 181-182; 187-top 189, Simbryo-cardiovascular system SUMMARY: The circulatory system, consisting of heart, blood vessels, and blood cells is the first functional organ to develop. This lecture will focus on the formation of the embryonic vasculature, the origin and formation of the early heart tube and primitive cardiac chambers, cardiac looping, and the primitive circulation. Between the 5th - 8th week of embryonic development, the tubular heart is remodeled into a four chambered structure. We will see how right and left atrioventricular canals connect each atrium with its respective ventricle, and how the atrial septum and definitive right and left atria form. We will also see why the great veins deliver blood to the right atrium while the pulmonary veins empty into the left. GLOSSARY: Angioblasts: precursors of blood vessels Angiogenesis: lengthening, branching, sprouting and remodeling of embryonic blood vessels Aortic arches: paired arteries surrounding the pharynx; portions will contribute to formation of the great arterial vessels Blood islands: clusters of cells in the yolk sac, connecting stalk and chorionic villi that form primitive blood vessels Cardiac jelly: gelatinous extracellular matrix that forms the middle layer of the heart tube Ductus venosus: shunts most of the blood in the umbilical vein into the inferior vena cava