Application No. AU 2019203067 A1 AUSTRALIAN PATENT OFFICE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Presence and Metabolism of Endogenous Androgenic–Anabolic Steroid Hormones in Meat-Producing Animals: a Review J
Food Additives and Contaminants Vol. 26, No. 5, May 2009, 640–671 Presence and metabolism of endogenous androgenic–anabolic steroid hormones in meat-producing animals: a review J. Scartha*, C. Akreb, L. van Ginkelc, B. Le Bizecd, H. De Brabandere, W. Korthf, J. Pointsg, P. Tealea and J. Kayh aHFL Sport Science (a Quotient Bioresearch Company), Fordham, UK; bCanadian Food Inspection Agency, Saskatoon, Canada; cNational Institute of Public Health and the Environment, RIVM, European Union Community Reference Laboratory for Residues, Bilthoven, the Netherlands; dLABERCA, Ecole Nationale Ve´te´rinaire de Nantes, Nantes, France; eFaculty of Veterinary Medicine, Laboratory of Chemical Analysis, Department of Veterinary Public Health and Food Safety, Ghent University, Merelbeke, Belgium; fNational Residue Survey, Australian Government Department of Agriculture, Fisheries and Forestry, Barton, ACT, Australia; gVeterinary Drugs Group, LGC, Teddington, UK; hVeterinary Medicines Directorate, Addlestone, UK (Received 17 April 2008; final version received 15 November 2008) The presence and metabolism of endogenous steroid hormones in meat-producing animals has been the subject of much research over the past 40 years. While significant data are available, no comprehensive review has yet been performed. Species considered in this review are bovine, porcine, ovine, equine, caprine and cervine, while steroid hormones include the androgenic–anabolic steroids testosterone, nandrolone and boldenone, as well as their precursors and metabolites. Information on endogenous steroid hormone concentrations is primarily useful in two ways: (1) in relation to pathological versus ‘normal’ physiology and (2) in relation to the detection of the illegal abuse of these hormones in residue surveillance programmes. Since the major focus of this review is on the detection of steroids abuse in animal production, the information gathered to date is used to guide future research. -

Jp Xvii the Japanese Pharmacopoeia
JP XVII THE JAPANESE PHARMACOPOEIA SEVENTEENTH EDITION Official from April 1, 2016 English Version THE MINISTRY OF HEALTH, LABOUR AND WELFARE Notice: This English Version of the Japanese Pharmacopoeia is published for the convenience of users unfamiliar with the Japanese language. When and if any discrepancy arises between the Japanese original and its English translation, the former is authentic. The Ministry of Health, Labour and Welfare Ministerial Notification No. 64 Pursuant to Paragraph 1, Article 41 of the Law on Securing Quality, Efficacy and Safety of Products including Pharmaceuticals and Medical Devices (Law No. 145, 1960), the Japanese Pharmacopoeia (Ministerial Notification No. 65, 2011), which has been established as follows*, shall be applied on April 1, 2016. However, in the case of drugs which are listed in the Pharmacopoeia (hereinafter referred to as ``previ- ous Pharmacopoeia'') [limited to those listed in the Japanese Pharmacopoeia whose standards are changed in accordance with this notification (hereinafter referred to as ``new Pharmacopoeia'')] and have been approved as of April 1, 2016 as prescribed under Paragraph 1, Article 14 of the same law [including drugs the Minister of Health, Labour and Welfare specifies (the Ministry of Health and Welfare Ministerial Notification No. 104, 1994) as of March 31, 2016 as those exempted from marketing approval pursuant to Paragraph 1, Article 14 of the Same Law (hereinafter referred to as ``drugs exempted from approval'')], the Name and Standards established in the previous Pharmacopoeia (limited to part of the Name and Standards for the drugs concerned) may be accepted to conform to the Name and Standards established in the new Pharmacopoeia before and on September 30, 2017. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0159073 A1 De Juan Et Al
US 20110159073A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0159073 A1 de Juan et al. (43) Pub. Date: Jun. 30, 2011 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA A6F 2/00 (2006.01) (US); Signe E. Varner, Los (52) U.S. Cl. ........................................................ 424/427 Angeles, CA (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Featured is a method for instilling one or more bioactive (21) Appl. No.: 12/981,038 agents into ocular tissue within an eye of a patient for the treatment of an ocular condition, the method comprising con (22) Filed: Dec. 29, 2010 currently using at least two of the following bioactive agent delivery methods (A)-(C): (A) implanting a Sustained release Related U.S. Application Data delivery device comprising one or more bioactive agents in a (63) Continuation of application No. 1 1/175,850, filed on posterior region of the eye so that it delivers the one or more Jul. 5, 2005, now abandoned. bioactive agents into the vitreous humor of the eye; (B) instill ing (e.g., injecting or implanting) one or more bioactive (60) Provisional application No. 60/585,236, filed on Jul. 2, agents Subretinally; and (C) instilling (e.g., injecting or deliv 2004, provisional application No. 60/669,701, filed on ering by ocular iontophoresis) one or more bioactive agents Apr. 8, 2005. into the vitreous humor of the eye. Patent Application Publication Jun. 30, 2011 Sheet 1 of 22 US 2011/O159073 A1 Patent Application Publication Jun. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act
Updated June 07, 2021 Bulk Drug Substances Nominated for Use in Compounding Under Section 503B of the Federal Food, Drug, and Cosmetic Act Three categories of bulk drug substances: • Category 1: Bulk Drug Substances Under Evaluation • Category 2: Bulk Drug Substances that Raise Significant Safety Risks • Category 3: Bulk Drug Substances Nominated Without Adequate Support Updates to Categories of Substances Nominated for the 503B Bulk Drug Substances List1 • Add the following entry to category 2 due to serious safety concerns of mutagenicity, cytotoxicity, and possible carcinogenicity when quinacrine hydrochloride is used for intrauterine administration for non- surgical female sterilization: 2,3 o Quinacrine Hydrochloride for intrauterine administration • Revision to category 1 for clarity: o Modify the entry for “Quinacrine Hydrochloride” to “Quinacrine Hydrochloride (except for intrauterine administration).” • Revision to category 1 to correct a substance name error: o Correct the error in the substance name “DHEA (dehydroepiandosterone)” to “DHEA (dehydroepiandrosterone).” 1 For the purposes of the substance names in the categories, hydrated forms of the substance are included in the scope of the substance name. 2 Quinacrine HCl was previously reviewed in 2016 as part of FDA’s consideration of this bulk drug substance for inclusion on the 503A Bulks List. As part of this review, the Division of Bone, Reproductive and Urologic Products (DBRUP), now the Division of Urology, Obstetrics and Gynecology (DUOG), evaluated the nomination of quinacrine for intrauterine administration for non-surgical female sterilization and recommended that quinacrine should not be included on the 503A Bulks List for this use. This recommendation was based on the lack of information on efficacy comparable to other available methods of female sterilization and serious safety concerns of mutagenicity, cytotoxicity and possible carcinogenicity in use of quinacrine for this indication and route of administration. -

CHAPTER I: INTRODUCTION Use of Steroids in Sports Such
CHAPTER I: INTRODUCTION The drugs are artificially derived from the main male hormone Testosterone. Testosterone is important for promoting and maintaining muscle growth and developing secondary male sex characteristics, such as a deepening voice and facial hair. Anabolic steroids, also called Anabolic-Androgenic Steroids (AASs), can build muscle and improve athletic performance, but they can also have significant adverse effects, especially when used incorrectly. Long-term, non-medical uses are linked to heart problems, unwanted physical changes, and aggression. There is growing concern worldwide about the non- medical use of steroids and its effects. Street names include Arnolds, gym candy, pumpers, roids, and stackers. AASs are synthetic versions of the primary male hormone, testosterone. They affect many parts of the body, including the muscles, bones, hair follicles, liver, kidneys, blood, immune system, reproductive system and the central nervous system. During puberty, increases in testosterone levels enable the development of characteristics such as facial and body hair growth, increased height and muscle mass, a deepening voice, and the sex drive. Testosterone can also contribute to competitiveness, self-esteem, and aggressiveness. Continuous use of AASs can lead to problems such as tolerance. They may even cause the body to stop producing its own testosterone. Some people use AASs continuously, but others try to minimize their possible adverse effects through different patterns of use. Use of Steroids In Sports Such As: 1. Cycling: The person takes AASs in cycles of 6 to 12 weeks known as the "on" period, followed by 4 weeks to several months off. 2. Stacking: Users combine several different types of steroids or incorporate other supplements in an attempt to maximize the effectiveness of the steroids. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, following to be employed in commercial fishing or the commercial UST, MXT, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0058896 A1 Dietrich Et Al
US 200400.58896A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0058896 A1 Dietrich et al. (43) Pub. Date: Mar. 25, 2004 (54) PHARMACEUTICAL PREPARATION (30) Foreign Application Priority Data COMPRISING AN ACTIVE DISPERSED ON A MATRIX Dec. 7, 2000 (EP)........................................ OO126847.3 (76) Inventors: Rango Dietrich, Konstanz (DE); Publication Classification Rudolf Linder, Kontanz (DE); Hartmut Ney, Konstanz (DE) (51) Int. Cl." ...................... A61K 31156; A61K 31/4439 (52) U.S. Cl. ........................... 514/171; 514/179; 514/338 Correspondence Address: (57) ABSTRACT NATH & ASSOCATES PLLC 1030 FIFTEENTH STREET, N.W. The present invention relates to the field of pharmaceutical SIXTH FLOOR technology and describes a novel advantageous preparation WASHINGTON, DC 20005 (US) for an active ingredient. The novel preparation is Suitable for 9 producing a large number of pharmaceutical dosage forms. (21) Appl. No.: 10/433,398 In the new preparation an active ingredient is present essentially uniformly dispersed in an excipient matrix com (22) PCT Filed: Dec. 6, 2001 posed of one or more excipients Selected from the group of fatty alcohol, triglyceride, partial glyceride and fatty acid (86) PCT No.: PCT/EPO1/14307 eSter. US 2004/0058896 A1 Mar. 25, 2004 PHARMACEUTICAL PREPARATION 0008 Further subject matters are evident from the claims. COMPRISING AN ACTIVE DISPERSED ON A MATRIX 0009. The preparations for the purpose of the invention preferably comprise numerous individual units in which at least one active ingredient particle, preferably a large num TECHNICAL FIELD ber of active ingredient particles, is present in an excipient 0001. The present invention relates to the field of phar matrix composed of the excipients of the invention (also maceutical technology and describes a novel advantageous referred to as active ingredient units hereinafter). -

Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2004) -- Supplement 1 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABACAVIR 136470-78-5 ACEXAMIC ACID 57-08-9 ABAFUNGIN 129639-79-8 ACICLOVIR 59277-89-3 ABAMECTIN 65195-55-3 ACIFRAN 72420-38-3 ABANOQUIL 90402-40-7 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABCIXIMAB 143653-53-6 ACITEMATE 101197-99-3 ABECARNIL 111841-85-1 ACITRETIN 55079-83-9 ABIRATERONE 154229-19-3 ACIVICIN 42228-92-2 ABITESARTAN 137882-98-5 ACLANTATE 39633-62-0 ABLUKAST 96566-25-5 ACLARUBICIN 57576-44-0 ABUNIDAZOLE 91017-58-2 ACLATONIUM NAPADISILATE 55077-30-0 ACADESINE 2627-69-2 ACODAZOLE 79152-85-5 ACAMPROSATE 77337-76-9 ACONIAZIDE 13410-86-1 ACAPRAZINE 55485-20-6 ACOXATRINE 748-44-7 ACARBOSE 56180-94-0 ACREOZAST 123548-56-1 ACEBROCHOL 514-50-1 ACRIDOREX 47487-22-9 ACEBURIC ACID 26976-72-7 -
FEP 5 Tier Rx Drug Formulary (607) Standard Option
FEP 5 Tier Rx Drug Formulary (607) Standard Option Effective July 1, 2021 The FEP formulary includes the preferred drug list which is comprised of Tier 1, generics and Tier 2, preferred brand-name drugs. Also included in the formulary are Tier 3, non-preferred brand-name drugs, Tier 4, preferred specialty drugs and Tier 5, non-preferred specialty drugs. Ask your physician if there is a generic drug available to treat your condition. If there is no generic drug available, ask your physician to prescribe a preferred brand-name drug. The preferred brand-name drugs within our formulary are listed to identify medicines that are clinically appropriate and cost-effective. Click on the category name in the Table of Contents below to go directly to that page INTRODUCTION ........................................................................................................................................................................................................................ 5 PREFACE ................................................................................................................................................................................................................................... 5 EXCLUDED DRUGS .................................................................................................................................................................................................................. 6 PRIOR APPROVAL .................................................................................................................................................................................................................. -
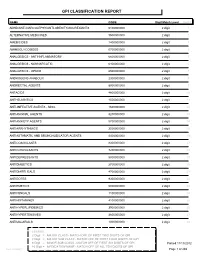
Gpi Drug Classification Report
GPI CLASSIFICATION REPORT NAME CODE Digit Match Level Count ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS 6100000000 2 digit 1 ALTERNATIVE MEDICINES 9500000000 2 digit 2 AMEBICIDES 1400000000 2 digit 3 AMINOGLYCOSIDES 0700000000 2 digit 4 ANALGESICS - ANTI-INFLAMMATORY 6600000000 2 digit 5 ANALGESICS - NONNARCOTIC 6400000000 2 digit 6 ANALGESICS - OPIOID 6500000000 2 digit 7 ANDROGENS-ANABOLIC 2300000000 2 digit 8 ANORECTAL AGENTS 8900000000 2 digit 9 ANTACIDS 4800000000 2 digit 10 ANTHELMINTICS 1500000000 2 digit 11 ANTI-INFECTIVE AGENTS - MISC. 1600000000 2 digit 12 ANTIANGINAL AGENTS 3200000000 2 digit 13 ANTIANXIETY AGENTS 5700000000 2 digit 14 ANTIARRHYTHMICS 3500000000 2 digit 15 ANTIASTHMATIC AND BRONCHODILATOR AGENTS 4400000000 2 digit 16 ANTICOAGULANTS 8300000000 2 digit 17 ANTICONVULSANTS 7200000000 2 digit 18 ANTIDEPRESSANTS 5800000000 2 digit 19 ANTIDIABETICS 2700000000 2 digit 20 ANTIDIARRHEALS 4700000000 2 digit 21 ANTIDOTES 9300000000 2 digit 22 ANTIEMETICS 5000000000 2 digit 23 ANTIFUNGALS 1100000000 2 digit 24 ANTIHISTAMINES 4100000000 2 digit 25 ANTIHYPERLIPIDEMICS 3900000000 2 digit 26 ANTIHYPERTENSIVES 3600000000 2 digit 27 ANTIMALARIALS 1300000000 2 digit 28 LEGEND: 2 Digit = MAJOR CLASS - MATCH OFF OF FIRST TWO DIGITS OF GPI 4 Digit = MAJOR SUB CLASS - MATCH OFF OF FIRST FOUR DIGITS OF GPI 6 Digit = MINOR SUB CLASS - MATCH OFF OF FIRST SIX DIGITS OF GPI Printed:11/13/2012 10 Digit = MEDICATION NAME - MATCH OFF OF ALL TEN DIGITS OF GPI Lee Cooper Page 1 of 265 NAME CODE Digit Match Level Count ANTIMYASTHENIC AGENTS