Variability in the Behavioural Responses of Three Generalist Herbivores to the Most Abundant Coumarin in Daphne Laureola Leaves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harper's Island Wetlands Butterflies & Moths (2020)
Introduction Harper’s Island Wetlands (HIW) nature reserve, situated close to the village of Glounthaune on the north shore of Cork Harbour is well known for its birds, many of which come from all over northern Europe and beyond, but there is a lot more to the wildlife at the HWI nature reserve than birds. One of our goals it to find out as much as we can about all aspects of life, both plant and animal, that live or visit HIW. This is a report on the butterflies and moths of HIW. Butterflies After birds, butterflies are probably the one of the best known flying creatures. While there has been no structured study of them on at HIW, 17 of Ireland’s 33 resident and regular migrant species of Irish butterflies have been recorded. Just this summer we added the Comma butterfly to the island list. A species spreading across Ireland in recent years possibly in response to climate change. Hopefully we can set up regular monitoring of the butterflies at HIW in the next couple of years. Butterfly Species Recorded at Harper’s Island Wetlands up to September 2020. Colias croceus Clouded Yellow Pieris brassicae Large White Pieris rapae Small White Pieris napi Green-veined White Anthocharis cardamines Orange-tip Lycaena phlaeas Small Copper Polyommatus icarus Common Blue Celastrina argiolus Holly Blue Vanessa atalanta Red Admiral Vanessa cardui Painted Lady Aglais io Peacock Aglais urticae Small Tortoiseshell Polygonia c-album Comma Speyeria aglaja Dark-green Fritillary Pararge aegeria Speckled Wood Maniola jurtina Meadow Brown Aphantopus hyperantus Ringlet Moths One group of insects that are rarely seen by visitors to HIW is the moths. -

Quantifying Dispersal in British Noctuid Moths
Quantifying dispersal in British noctuid moths Hayley Bridgette Clarke Jones Doctor of Philosophy University of York Biology September 2014 1 Abstract Dispersal is an important process in the ecology and evolution of organisms, affecting species’ population dynamics, gene flow, and range size. Around two thirds of common and widespread British macro-moths have declined in abundance over the last 40 years, and dispersal ability may be important in determining whether or not species persist in this changing environment. However, knowledge of dispersal ability in macro-moths is lacking because dispersal is difficult to measure directly in nocturnal flying insects. This thesis investigated the dispersal abilities of British noctuid moths to examine how dispersal ability is related to adult flight morphology and species’ population trends. Noctuid moths are an important taxon to study because of their role in many ecosystem processes (e.g. as pollinators, pests and prey), hence their focus in this study. I developed a novel tethered flight mill technique to quantify the dispersal ability of a range of British noctuid moths (size range 12 – 27 mm forewing length). I demonstrated that this technique provided measures of flight performance in the lab (measures of flight speed and distance flown overnight) that reflected species’ dispersal abilities reported in the wild. I revealed that adult forewing length was a good predictor of inter- specific differences in flight performance among 32 noctuid moth species. I also found high levels of intra-specific variation in flight performance, and both adult flight morphology and resource-related variables (amount of food consumed by individuals prior to flight, mass loss by adults during flight) contributed to this variation. -

Database of Irish Lepidoptera. 1 - Macrohabitats, Microsites and Traits of Noctuidae and Butterflies
Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Irish Wildlife Manuals No. 35 Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies Ken G.M. Bond and Tom Gittings Department of Zoology, Ecology and Plant Science University College Cork Citation: Bond, K.G.M. and Gittings, T. (2008) Database of Irish Lepidoptera. 1 - Macrohabitats, microsites and traits of Noctuidae and butterflies. Irish Wildlife Manual s, No. 35. National Parks and Wildlife Service, Department of the Environment, Heritage and Local Government, Dublin, Ireland. Cover photo: Merveille du Jour ( Dichonia aprilina ) © Veronica French Irish Wildlife Manuals Series Editors: F. Marnell & N. Kingston © National Parks and Wildlife Service 2008 ISSN 1393 – 6670 Database of Irish Lepidoptera ____________________________ CONTENTS CONTENTS ........................................................................................................................................................1 ACKNOWLEDGEMENTS ....................................................................................................................................1 INTRODUCTION ................................................................................................................................................2 The concept of the database.....................................................................................................................2 The structure of the database...................................................................................................................2 -
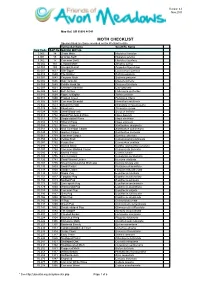
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -
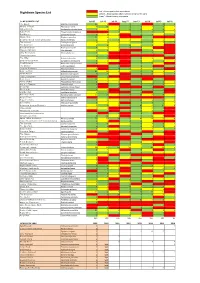
Highdown Species List 2008 and 2009 and 2010 and 2011 and 2012 and 2013 and 2014 and 2015 and 2016.Xlsx
'red' = those species that were absent Highdown Species List 'yellow' = those species who's numbers remained the same 'green' = those showing an increase CORE SURVEY LIST Jul‐09 Jul‐10 Jul‐11 Aug‐12 Jun‐13 Jul‐14 Jul‐15 Jul‐16 The Snout Hypena proboscidalis 1142322 Mother of Pearl Pleuroptya ruralis 911334242 Willow Beauty Peribatodes rhomboidaria 76368491 Ruby Tiger Phragmatobia fuliginosa 212232 Buff Ermine Spilosoma luteum 862 2913 Muslin Moth Diaphora mendica 12 6 2 1 Broad-bordered Yellow Underwing Noctua fimbriata 52621311 The Coronet Craniophora ligustri 43114725 The Sycamore Acronicta aceris 2323232 Burnished Brass Diachrysia chrysitis 632332 Chinese Character Cilix glaucata 131 21 Grey Pine Carpet Thera obeliscata 74 333 Carcina quercana 23 1 The Miller Acronicta leporine 68 642 Swallow-tailed Moth Ourapteryx sambucaria 951 6243 Small Emerald Hemistola chrysoprasaria 81 232 The Drinker Euthrix potatoria 1122 Swallow Prominent Pheosia tremula 4 34212 Rosy Footman Miltochrista miniata 4118224946 White Ermine Spilosoma lubricipeda 791 3623 True Lover's Knot Lycophotia porphyrea 1 Knot Grass Acronicta rumicis 127352 Angle Shades Phlogophora meticulosa 84114121 Nut-tree Tussock Colocasia coryli 4 4 3341 Brown-tail Euproctis chrysorrhoea 72 24 Blood-vein Timandra comae 72 1112 Slender Pug Eupithecia tenuiata 24 5 1 Brimstone Moth Opisthograptis luteolata 11 19 2 7 12 6 6 4 Purple Thorn Selenia tetralunaria 1 2 Peppered Moth Biston betularia 75126253 Buff Arches Habrosyne pyritoides 532 4464 Setaceous Hebrew Character Xestia c‐nigrum -

Second Generation Sequencing and Morphological Faecal Analysis
Hope et al. Frontiers in Zoology 2014, 11:39 http://www.frontiersinzoology.com/content/11/1/39 RESEARCH Open Access Second generation sequencing and morphological faecal analysis reveal unexpected foraging behaviour by Myotis nattereri (Chiroptera, Vespertilionidae) in winter Paul R Hope1,2*†, Kristine Bohmann1,3†, M Thomas P Gilbert3, Marie Lisandra Zepeda-Mendoza3, Orly Razgour1 and Gareth Jones1 Abstract Background: Temperate winters produce extreme energetic challenges for small insectivorous mammals. Some bat species inhabiting locations with mild temperate winters forage during brief inter-torpor normothermic periods of activity. However, the winter diet of bats in mild temperate locations is studied infrequently. Although microscopic analyses of faeces have traditionally been used to characterise bat diet, recently the coupling of PCR with second generation sequencing has offered the potential to further advance our understanding of animal dietary composition and foraging behaviour by allowing identification of a much greater proportion of prey items often with increased taxonomic resolution. We used morphological analysis and Illumina-based second generation sequencing to study the winter diet of Natterer’sbat(Myotis nattereri) and compared the results obtained from these two approaches. For the first time, we demonstrate the applicability of the Illumina MiSeq platform as a data generation source for bat dietary analyses. Results: Faecal pellets collected from a hibernation site in southern England during two winters (December-March 2009–10 and 2010–11), indicated that M. nattereri forages throughout winter at least in a location with a mild winter climate. Through morphological analysis, arthropod fragments from seven taxonomic orders were identified. A high proportion of these was non-volant (67.9% of faecal pellets) and unexpectedly included many lepidopteran larvae. -

Moths201707.Pdf
Friends of Sheskinmore Moth Species List Key o Months when moth is on the wing Red List Species under threat of extinction in Ireland Data from Ralph Sheppard and Angus Tyner Macro-moths in the blue section; Micro-moths in the green section Common name Scientific Name Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Recorded Notes Angle Shades Phlogophora meticulosa o o o o o o o o Jul-05 Anomalous Stilbia anomala o o Jul-05 Antler Moth Cerapteryx graminis o o Jul-05 Archer's Dart Agrotis vestigialis o o o o Jul-05 Argent & Sable Rheumaptera hastata o o Jun-05 Red List August Thorn Ennomos quercinaria o o o Jul-05 Autumn Green Carpet Chloroclysta miata o o o o o Jul-05 Autumnal Rustic Eugnorisma glareosa o o o Jul-05 Barred Straw Eulithis pyraliata o o Jul-05 Barred Umber Plagodis pulveraria o o Jul-05 Barred Yellow Cidaria fulvata o o o Jul-05 Beautiful Brocade Lacanobia contigua o o o Jul-05 Beautiful Golden Y Autographa pulchrina o o Jul-05 Black Rustic Aporophyla nigra o o Jul-05 Bordered Pug Eupithecia succenturiata o o Jul-05 Bright-line Brown-eye Lacanobia oleracea o o o Jul-05 Brimstone Moth Opisthograptis luteolata o o o o o Jul-05 Broad-bordered Yellow Noctua fimbriata o o o Jun-05 Underwing Broom Moth Melanchra pisi o o o Jul-05 Brown Silver-line Petrophora chlorosata o o Jul-05 Brown-line Bright Eye Mythimna conigera o o Jul-05 Buff Arches Habrosyne pyritoides o o Jul-05 Buff Ermine Spilosoma luteum o o Jul-05 Buff-tip Phalera bucephala o o o Jul-05 Burnished Brass Diachrysia chrysitis o o o Jul-05 Cabbage Moth Mamestra brassicae -

Moths Trapped at Hilfield Friary 2017-2018
MOTHS TRAPPED AT HILFIELD FRIARY 2017-2018 2013 CL No 2000 B No English Name Scientific Name Date 1 Date 2 Date 3 Date 4 Date 5 Date 6 15.010 288 Caloptilia stigmatella 23/10/2018 16.001 424 Bird Cherry Ermine Yponomeuta evonymella 20/07/2018 18.001 464 Diamond-back Moth Plutella xylostella 14/08/2018 28.010 647 Brown House Moth Hofmannophila pseudospretella 14/08/2018 35.085 762 Athrips mouffetella 15/07/2018 41.002 873 Blastobasis adustella 14/08/2018 49.004 1010 Red-barred Tortrix Ditula angustiorana 20/07/2018 49.024 969 Chequered Fruit-tree Tortrix Pandemis corylana 14/08/2018 49.025 970 Barred Fruit-tree Tortrix Pandemis cerasana 12/06/2018 49.031 989 Timothy Tortrix Aphelia paleana 12/06/2018 49.039 998 Light Brown Apple Moth Epiphyas postvittana 12/06/2018 23/10/2018 49.065 1039 Acleris comariana/laterana 14/08/2018 49.077 1048 Garden Rose Tortrix Acleris variegana 14/08/2018 49.080 1053 Acleris hastiana 23/10/2018 49.166 1076 Celypha lacunana 12/06/2018 14/08/2018 49.292 1174 Notocelia cynosbatella 15/07/2018 49.294 1175 Bramble-shoot Moth Notocelia uddmanniana 12/06/2018 49.338 1261 Codling Moth Cydia pomonella 20/07/2018 14/08/2018 62.048 1470 Euzophera pinguis 20/07/2018 63.017 1377 Anania lancealis 15/07/2018 63.025 1376 Small Magpie Anania hortulata 12/06/2018 20/07/2018 63.031 1395 Rusty Dot Pearl Udea ferrugalis 20/07/2018 63.034 1390 Udea prunalis 20/07/2018 63.037 1392 Udea olivalis 12/06/2018 63.038 1405 Mother of Pearl Pleuroptya ruralis 20/07/2018 14/08/2018 63.067 1338 Eudonia lacustrata 14/08/2018 63.080 1293 Garden -

The Potential Impacts of Climate Change on the Biodiversity of Norfolk Jeff Price
The potential impacts of climate change on the biodiversity of Norfolk Jeff Price Introduction on a trajectory for ~3.2°C increase (UNEP Climate change is posing, and will continue 2016). While this is an improvement over to pose, increasing risks to biodiversity the previous ‘business as usual’ estimate (O’Neill et al. 2017). Changes in phenology of 4°- 4.5°C, it is still likely to have a large and range were first noted more than a impact on biodiversity. decade ago (Root et al. 2003) with many This paper reviews the projected climate publications since. Land use change is change impacts (relative to 1961-1990 increasingly a problem as species are being baseline) on some of the biodiversity further challenged by barriers to their in Norfolk (including birds, mammals, potential dispersal with their preferred reptiles, amphibians, butterflies, common climate across fragmented landscapes macro moths, dragonflies, bumblebees, (Settele et al. 2014). Many studies have grasshoppers, shieldbugs, ferns, orchids, examined the potential future impacts and some trees and shrubs. The paper of climate change on biodiversity using concentrates on the species currently found a variety of modelling techniques. This in Norfolk (largely based on lists on the includes results from Wallace Initiative Norfolk and Norwich Naturalist’s Society Phase 1 models showing the potential for website) and not on potential colonists range losses of greater than 50% across large from Europe. The exception is for some fractions of species globally at warming of the birds and dragonflies. For brevity levels of approximately 3.6 °C above pre- it concentrates on the climate changes industrial levels (Warren et al. -

BIODIVERSITY and ENVIRONMENT of NEW ROAD, LITTLE LONDON and NEIGHBOURING COUNTRYSIDE by Dr Paul Sterry Contents: 1
BIODIVERSITY AND ENVIRONMENT OF NEW ROAD, LITTLE LONDON AND NEIGHBOURING COUNTRYSIDE by Dr Paul Sterry Contents: 1. Summary. 2. A brief history. 3. Notable habitats alongside New Road and in the neighbouring countryside. 4. Protected and notable species found on New Road and in the surrounding countryside. Appendix 1 - Historical land use in Little London and its influence on biodiversity. Appendix 2 - Lepidoptera (Butterflies and Moths) recorded on New Road, Little London 2004-2019 (generalised OS Grid Reference SU6159). Appendix 3 - Ageing Hedgerows. About the author : Paul Sterry has BSc and PhD in Zoology and Ecology from Imperial College, London. After 5 years as a Research Fellow at the University of Sussex working on freshwater ecology he embarked on a freelance career as a wildlife author and photographer. Over the last 35 years he has written and illustrated more than 50 books, concentrating mainly on British Wildlife, with the emphasis on photographic field guides. Best-selling titles include Collins Complete British Trees, Collins Complete British Wildlife and Collins Life-size Birds. Above: Barn Owl flying over grassland in the neighbourhood of New Road. 1. Summary Located in the Parish of Pamber, Little London is a Biodiversity hotspot with New Road at its environmental heart. Despite the name New Road is one of the oldest highways in the village and this is reflected in the range of wildlife found along its length, and in the countryside bordering it. New Road has significance for wildlife far beyond is narrow, single-track status. Its ancient hedgerows and adjacent meadows are rich in wildlife but of equal importance is its role as a corridor of wildlife connectivity. -

Zur Verbreitung Der Bandeule Noctua Janthe BKH. (Lep., Noctuidae) in Den Östlichen Bundesländern Deutschlands )
© Entomologische Nachrichten und Berichte; downloadEntomologische unter www.biologiezentrum.at Nachrichten und Berichte, 38,1994/4 221 W. HEINICKE, Gera Zur Verbreitung der Bandeule Noctua janthe BKH. (Lep., Noctuidae) in den östlichen Bundesländern Deutschlands ) Summary An overview of the occurence of Noctua janthe BKH. in the eastern states of the Federal Republic of Germany is provided; it is based on 79 specimens in 46 collections. Noctua janthe is known from Thüringen, Sach sen, Sachsen-Anhalt and Mecklenburg-Vorpommern; it was not found, so far, in Brandenburg, nor in Berlin. The eastern distributional limit of the species is discussed and the question is asked whether Noctua janthe should be regarded as an expanding species. Detailed reasons for this interpretation are presented. Résumé On y donne un aperçu sur la distribution de Noctua janthe BKH. dans les Etats fédérales orientales de l’Allemagne, basé sur 79 preuves dans 46 collections. Jusqu’ici Noctua janthe était connu de la Thuringe, de la Saxe, Saxe-Anhalt et Mecklenburg-Vorpommern. On n’a pas encore trouvé cette espèce en Brandenburg et Berlin. L’au teur discusse la ligne de la frontière orientale de l’aire de Noctua janthe et en pose la question s’il devrait considérer cette espèce comme espèce d’expansion. Les raisons de cette considération sont expliquées en détail. 1. Problemstellung und Untersuchungsergebnisse 1:25000 ist in Abb. 1 dargestellt. Im einzelnen stammen die Tiere aus folgenden Gebieten (vereinfachte Doku Vor drei Jahren erhoben v. MENTZER, MOBERG & mentation der Daten): FIBIGER (1991 a, b) das Taxon Noctua janthe (BORK- HAUSEN, 1792) in den Rang einer selbständigen Art. -

Und Noctua Tertia (V.M E N T Z E R , M O Berg & F Ib Ig E R, 1991) Im Komplex, Janthina“ (Lep., Noctuidae)
© Entomologische Nachrichten und Berichte; downloadEntomologische unter www.biologiezentrum.at Nachrichten und Berichte, 49,2005/1 33 R. Plontke , Magdala, E. Friedrich , K. G rajetzki , F. H ünefeld , R. M üller , alle Jena & W. H einicke , Gera Zweifel an der Artberechtigung vonNoctua janthe (B o r k h a u se n , 1792) und Noctua tertia (v.M e n t z e r , M o berg & F ib ig e r, 1991) im Komplex, janthina“ (Lep., Noctuidae) Zusammenfassung Fl -Zuchten aus Eiablagen von Weibchen mit dem Erscheinungsbild , Janthe“ und „ tertia “ zeigten, dass deren Nachkommen ausschließlich die Merkmalskombination von Janthina“ tragen. Diese Tatsache veranlasst die Autoren, die vermutete Artberechtigung dreier Arten im Komplex Janthina“ anzuzweifeln und alle drei als Formen einer einzigen Art anzusehen. Summary Doubts about the specific distinctness of Noctua janthe (B orkhausen , 1792) und Noctua tertia (v. M entzer , M oberg & F ibiger, 1991) within the “ja n th in a” complex (Lep., Noctuidae). - Eggs of females exhibiting “ j a n t h eand “tertia"- type patterns produces exclusively offspring of “janthina” appearance. Therefore, the authors doubt the presumed specific distinctness of the three different taxa who instead regard them as morphs of one single species. 1. Vorgeschichte janthina-Raupe abwichen. Mit sehr vorsichtigen Wor ten „wage er hier eine neue Art aufzustellen die er Seit vielen Jahren ist es eines der Anliegen der Fach wegen der großen Ähnlichkeit zu janthina als janthe gruppe Entomologie Jena im Thüringer Entomologen benannte. Aufgrund der Tatsache, dass er aus Funden verband e .V ., Schmetterlinge zu züchten. Besonderes ebensolcher Raupen aber immer nur Falter mit dem Augenmerk wird dabei verständlicherweise auf Tiere Aussehen von janthina erhalten hat, zog B orkhausen gelegt, die selten sind, über deren Biologie wenig be die Artberechtigung von janthe später wieder zurück.