Second Generation Sequencing and Morphological Faecal Analysis Reveal Unexpected Foraging Behaviour by Myotis Nattereri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic Paper Chilterns Beechwoods
. O O o . 0 O . 0 . O Shoping growth in Docorum Appendices for Topic Paper for the Chilterns Beechwoods SAC A summary/overview of available evidence BOROUGH Dacorum Local Plan (2020-2038) Emerging Strategy for Growth COUNCIL November 2020 Appendices Natural England reports 5 Chilterns Beechwoods Special Area of Conservation 6 Appendix 1: Citation for Chilterns Beechwoods Special Area of Conservation (SAC) 7 Appendix 2: Chilterns Beechwoods SAC Features Matrix 9 Appendix 3: European Site Conservation Objectives for Chilterns Beechwoods Special Area of Conservation Site Code: UK0012724 11 Appendix 4: Site Improvement Plan for Chilterns Beechwoods SAC, 2015 13 Ashridge Commons and Woods SSSI 27 Appendix 5: Ashridge Commons and Woods SSSI citation 28 Appendix 6: Condition summary from Natural England’s website for Ashridge Commons and Woods SSSI 31 Appendix 7: Condition Assessment from Natural England’s website for Ashridge Commons and Woods SSSI 33 Appendix 8: Operations likely to damage the special interest features at Ashridge Commons and Woods, SSSI, Hertfordshire/Buckinghamshire 38 Appendix 9: Views About Management: A statement of English Nature’s views about the management of Ashridge Commons and Woods Site of Special Scientific Interest (SSSI), 2003 40 Tring Woodlands SSSI 44 Appendix 10: Tring Woodlands SSSI citation 45 Appendix 11: Condition summary from Natural England’s website for Tring Woodlands SSSI 48 Appendix 12: Condition Assessment from Natural England’s website for Tring Woodlands SSSI 51 Appendix 13: Operations likely to damage the special interest features at Tring Woodlands SSSI 53 Appendix 14: Views About Management: A statement of English Nature’s views about the management of Tring Woodlands Site of Special Scientific Interest (SSSI), 2003. -

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Catalogue2013 Web.Pdf
bwfp British Wild Flower Plants www.wildflowers.co.uk Plants for Trade Plants for Home Specialist Species Wildflower Seed Green Roof Plants Over 350 species Scan here to of British native buy online plants 25th Anniversary Year Finding Us British Wild Flower Plants Burlingham Gardens 31 Main Road North Burlingham Norfolk NR13 4TA Phone / Fax: (01603) 716615 Email: [email protected] Website: http://www.wildflowers.co.uk Twitter: @WildflowersUK Nursery Opening Times Monday to Thursday: 10.00am - 4.00pm Friday: 10.00am - 2.30pm Please note that we are no longer open at weekends or Bank Holidays. Catalogue Contents Contact & Contents Page 02 About Us Page 03 Mixed Trays Pages 04-05 Reed Beds Page 06 Green Roofs Page 07 Wildflower Seeds Page 08 Planting Guide Pages 09-10 Attracting Wildlife Page 11 Rabbit-Proof Plants Page 12 List of Plants Pages 13-50 Scientific Name Look Up Pages 51-58 Terms & Conditions Page 59 www.wildflowers.co.uk 2 Tel/Fax:(01603)716615 About Us Welcome.... About Our Plants We are a family-run nursery, situated in Norfolk on a Our species are available most of the year in: six acre site. We currently stock over 350 species of 3 native plants and supply to all sectors of the industry Plugs: Young plants in 55cm cells with good rootstock. on a trade and retail basis. We are the largest grower of native plants in the UK and possibly Europe. Provenance Our species are drawn from either our own seed collections or from known provenance native sources. We comply with the Flora Locale Code of Practice. -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Millichope Park and Estate Invertebrate Survey 2020
Millichope Park and Estate Invertebrate survey 2020 (Coleoptera, Diptera and Aculeate Hymenoptera) Nigel Jones & Dr. Caroline Uff Shropshire Entomology Services CONTENTS Summary 3 Introduction ……………………………………………………….. 3 Methodology …………………………………………………….. 4 Results ………………………………………………………………. 5 Coleoptera – Beeetles 5 Method ……………………………………………………………. 6 Results ……………………………………………………………. 6 Analysis of saproxylic Coleoptera ……………………. 7 Conclusion ………………………………………………………. 8 Diptera and aculeate Hymenoptera – true flies, bees, wasps ants 8 Diptera 8 Method …………………………………………………………… 9 Results ……………………………………………………………. 9 Aculeate Hymenoptera 9 Method …………………………………………………………… 9 Results …………………………………………………………….. 9 Analysis of Diptera and aculeate Hymenoptera … 10 Conclusion Diptera and aculeate Hymenoptera .. 11 Other species ……………………………………………………. 12 Wetland fauna ………………………………………………….. 12 Table 2 Key Coleoptera species ………………………… 13 Table 3 Key Diptera species ……………………………… 18 Table 4 Key aculeate Hymenoptera species ……… 21 Bibliography and references 22 Appendix 1 Conservation designations …………….. 24 Appendix 2 ………………………………………………………… 25 2 SUMMARY During 2020, 811 invertebrate species (mainly beetles, true-flies, bees, wasps and ants) were recorded from Millichope Park and a small area of adjoining arable estate. The park’s saproxylic beetle fauna, associated with dead wood and veteran trees, can be considered as nationally important. True flies associated with decaying wood add further significant species to the site’s saproxylic fauna. There is also a strong -

Diversity and Resource Choice of Flower-Visiting Insects in Relation to Pollen Nutritional Quality and Land Use
Diversity and resource choice of flower-visiting insects in relation to pollen nutritional quality and land use Diversität und Ressourcennutzung Blüten besuchender Insekten in Abhängigkeit von Pollenqualität und Landnutzung Vom Fachbereich Biologie der Technischen Universität Darmstadt zur Erlangung des akademischen Grades eines Doctor rerum naturalium genehmigte Dissertation von Dipl. Biologin Christiane Natalie Weiner aus Köln Berichterstatter (1. Referent): Prof. Dr. Nico Blüthgen Mitberichterstatter (2. Referent): Prof. Dr. Andreas Jürgens Tag der Einreichung: 26.02.2016 Tag der mündlichen Prüfung: 29.04.2016 Darmstadt 2016 D17 2 Ehrenwörtliche Erklärung Ich erkläre hiermit ehrenwörtlich, dass ich die vorliegende Arbeit entsprechend den Regeln guter wissenschaftlicher Praxis selbständig und ohne unzulässige Hilfe Dritter angefertigt habe. Sämtliche aus fremden Quellen direkt oder indirekt übernommene Gedanken sowie sämtliche von Anderen direkt oder indirekt übernommene Daten, Techniken und Materialien sind als solche kenntlich gemacht. Die Arbeit wurde bisher keiner anderen Hochschule zu Prüfungszwecken eingereicht. Osterholz-Scharmbeck, den 24.02.2016 3 4 My doctoral thesis is based on the following manuscripts: Weiner, C.N., Werner, M., Linsenmair, K.-E., Blüthgen, N. (2011): Land-use intensity in grasslands: changes in biodiversity, species composition and specialization in flower-visitor networks. Basic and Applied Ecology 12 (4), 292-299. Weiner, C.N., Werner, M., Linsenmair, K.-E., Blüthgen, N. (2014): Land-use impacts on plant-pollinator networks: interaction strength and specialization predict pollinator declines. Ecology 95, 466–474. Weiner, C.N., Werner, M , Blüthgen, N. (in prep.): Land-use intensification triggers diversity loss in pollination networks: Regional distinctions between three different German bioregions Weiner, C.N., Hilpert, A., Werner, M., Linsenmair, K.-E., Blüthgen, N. -

University of Copenhagen, Copenhagen, Denmark
Bucking the trend The diversity of Anthropocene 'winners' among British moths Boyes, Douglas H.; Fox, Richard; Shortall, Chris R.; Whittaker, Robert J. Published in: Frontiers of Biogeography DOI: 10.21425/F5FBG43862 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Boyes, D. H., Fox, R., Shortall, C. R., & Whittaker, R. J. (2019). Bucking the trend: The diversity of Anthropocene 'winners' among British moths. Frontiers of Biogeography, 11(3), 1-15. [3862]. https://doi.org/10.21425/F5FBG43862 Download date: 28. sep.. 2021 UC Merced Frontiers of Biogeography Title Bucking the trend: the diversity of Anthropocene ‘winners’ among British moths Permalink https://escholarship.org/uc/item/6hz0x33v Journal Frontiers of Biogeography, 11(3) Authors Boyes, Douglas H. Fox, Richard Shortall, Chris R. et al. Publication Date 2019 DOI 10.21425/F5FBG43862 Supplemental Material https://escholarship.org/uc/item/6hz0x33v#supplemental License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California a Frontiers of Biogeography 2019, 11.3, e43862 Frontiers of Biogeography RESEARCH ARTICLE the scientific journal of the International Biogeography Society Bucking the trend: the diversity of Anthropocene ‘winners’ among British moths Douglas H. Boyes1,*, Richard Fox2, Chris R. Shortall3 and Robert J. Whittaker1,4 1 School of Geography and the Environment, University of Oxford, South Parks Road, Oxford, OX1 3QY, UK; 2 Butterfly Conservation, Manor Yard, East Lulworth, Dorset, BH20 5QP, UK; 3 Rothamsted Insect Survey, Rothamsted Research, Harpenden AL5 2JQ, UK and 4 Center for Macroecology, Evolution and Climate, GLOBE Institute, University of Copenhagen, Copenhagen, Denmark. -

A Sur Hamp Peter 25 Ju Autho Rvey of Th Pton Brow Borough Ne 2015
A survey of the inverttebrates of the Hampton brownfield study site, Peterborough 25 June 2015 Authors: Buglife and BSG Ecology BLANK PAGE Acknowledgements: Buglife and BSG would like to thank O&H Hampton Ltd for undertaking the habitat creation work and providing access and support Report title A survey of the invertebrates of the Hampton brownfield study site, Peterborough Draft version/final FINAL File reference OH Hampton Draft Report_Final_240715 Buglife - The Invertebrate Conservation Trust is a registered charity at Bug House, Ham Lane, Orton Waterville, Peterborough, PE2 5UU Company no. 4132695, Registered charity no. 1092293, Scottish charity no. SC04004 BSG Ecology - Registered in: England and Wales | No. OC328772 | Registered address: Wyastone Business Park, Monmouth, NP25 3SR Contents 1 Summary ....................................................................................................................................................... 2 2 Introduction .................................................................................................................................................... 3 3 Site Description ............................................................................................................................................. 4 4 Methods ......................................................................................................................................................... 9 5 Results ........................................................................................................................................................ -

Sambia Succinica, a Crown Group Tenthredinid from Eocene Baltic Amber (Hymenoptera: Tenthredinidae)
Insect Systematics & Evolution 43 (2012) 271–281 brill.com/ise Sambia succinica, a crown group tenthredinid from Eocene Baltic amber (Hymenoptera: Tenthredinidae) Lars Vilhelmsena,* and Michael S. Engelb aNatural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark bDivision of Entomology (Paleoentomology), Natural History Museum and Department of Ecology & Evolutionary Biology, 1501 Crestline Drive, Suite 140, University of Kansas, Lawrence KS 66045, USA *Corresponding author, e-mail: [email protected] Published 17 December 2012 Abstract Sambia succinica gen. et sp.n. from Eocene Baltic amber is described and illustrated. It is apparently the first amber fossil that can be definitively assigned to Tenthredininae. It displays two diagnostic forewing characters for this subfamily: having a bend distally in vein R and the junctions of veins M and Rs + M with vein R being some distance from each other. The variance and possible transitions between the anal vein configurations among the genera in Tenthredininae is briefly discussed. Keywords amber inclusion, sawfly, Tertiary, Eocene, taxonomy Introduction Tenthredinidae is the largest family of non-apocritan Hymenoptera by far, comprising more than 5500 described species (Huber 2009; Taeger & Blank 2010). Together with five other families they comprise the Tenthredinoidea or true sawflies. The larvae of the members of the superfamily are all herbivores and most are external feeders on green parts of angiosperms; however, other host plants and feeding modes (e.g., leafrolling, leafmining, or galling in leaves, buds and shoots; see Nyman et al. 1998, 2000) do occur. Recent comprehensive treatments of the phylogeny of the basal hymenopteran lineages, while providing strong support for the Tenthredinoidea, have consistently failed to retrieve the Tenthredinidae as monophyletic (Vilhelmsen 2001; Schulmeister 2003; Ronquist et al. -

Garden Moth Scheme Report 2016
Garden Moth Scheme Report 2016 Heather Young – April 2017 1 GMS Report 2016 CONTENTS PAGE Introduction 2 Top 30 species 2016 3 Population trends (?) of our commonest garden moths 5 Autumn Moths 12 Winter GMS 2016-17 14 Antler Moth infestations 16 GMS Annual Conference 2017 19 GMS Sponsors 20 Links & Acknowledgements 21 Cover photograph: Fan-foot (R. Young) Introduction The Garden Moth Scheme (GMS) welcomes participants from all parts of the United Kingdom and Ireland, and in 2016 received 341 completed recording forms, slightly fewer than last year (355). The scheme is divided into 12 regions, monitoring 233 species of moth in every part of the UK and Ireland (the ‘Core Species’), along with a variable number of ‘Regional Species’. For each of the last seven years, we have had records from over 300 sites across the UK and Ireland, and later in the report there are a series of charts representing the population trends (or fluctuations) of our most abundant species over this period. The database has records dating back to 2003 when the scheme began in the West Midlands, and now contains over 1 ¼ million records, providing a very valuable resource to researchers. Scientists and statisticians from Birmingham and Manchester Universities are amongst those interested in using our data, as well as the ongoing research being undertaken by the GMS’s own John Wilson. There is an interesting follow-up article by Evan Lynn on the Quarter 4 GMS newsletter piece by Duncan Brown on Antler Moth infestations, and a report on the very successful 2017 Annual Conference in Apperley Village Hall, near Tewkesbury. -

Cogges Link Road Terrestrial Invertebrate Survey
Oxfordshire Highways Cogges Link Road Terrestrial Invertebrate Survey B0834600/Doc/CLR/23 April 2008 Jacobs Engineering UK Ltd, Jacobs House, 427 London Road, Reading, Berkshire, RG6 1BL UK Tel 0118 963 5000 Fax 0118 949 1054 Copyright Jacobs Engineering UK Ltd. All rights reserved No part of this report may be copied or reproduced by any means without prior written permission from Jacobs Engineering UK Ltd. If you have received this report in error, please destroy all copies in your possession or control and notify Jacobs Engineering UK Ltd. This report has been prepared for the exclusive use of the commissioning party and unless otherwise agreed in writing by Jacobs Engineering UK Ltd, no other party may use, make use of or rely on the contents of the report. If others choose to rely upon this report they do so entirely at their own risk. No liability is accepted by Jacobs Engineering UK Ltd for any use of this report, other than for the purposes for which it was originally prepared and provided. Opinions and information provided in the report are on the basis of Jacobs Engineering UK Ltd using due skill, care and diligence in the preparation of the same and no explicit warranty is provided as to their accuracy. It should be noted and it is expressly stated that no independent verification of any of the documents or information supplied to Jacobs Engineering UK Ltd has been made. Oxfordshire Highways Cogges Link Road Aquatic Invertebrate Survey Document No : B0834600/Doc/PA/CLR/23 Revision No : 01 Date : April 2008 Prepared by : Sarah Jennings Checked by : Michael Jennings Approved by : Jon Mullins NB This document supersedes Doc No. -
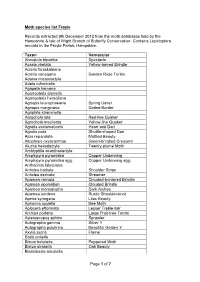
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera