Soltis Apps5
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Lemur News 7 (2002).Pdf
Lemur News Vol. 7, 2002 Page 1 Conservation International’s President EDITORIAL Awarded Brazil’s Highest Honor In recognition of his years of conservation work in Brazil, CI President Russell Mittermeier was awarded the National Are you in favor of conservation? Do you know how conser- Order of the Southern Cross by the Brazilian government. vation is viewed by the academic world? I raise these ques- Dr. Mittermeier received the award on August 29, 2001 at tions because they are central to current issues facing pri- the Brazilian Ambassador's residence in Washington, DC. matology in general and prosimians specifically. The National Order of the Southern Cross was created in The Duke University Primate Center is in danger of being 1922 to recognize the merits of individuals who have helped closed because it is associated with conservation. An inter- to strengthen Brazil's relations with the international com- nal university review in 2001 stated that the Center was too munity. The award is the highest given to a foreign national focused on conservation and not enough on research. The re- for service in Brazil. viewers were all researchers from the "hard" sciences, but For the past three decades, Mittermeier has been a leader in they perceived conservation to be a negative. The Duke ad- promoting biodiversity conservation in Brazil and has con- ministration had similar views and wanted more emphasis ducted numerous studies on primates and other fauna in the on research and less on conservation. The new Director has country. During his time with the World Wildlife Fund three years to make that happen. -

Early Evolution of the Angiosperm Clade Asteraceae in the Cretaceous of Antarctica
Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica Viviana D. Barredaa,1,2, Luis Palazzesia,b,1, Maria C. Telleríac, Eduardo B. Oliverod, J. Ian Rainee, and Félix Forestb aDivisión Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Consejo Nacional de Investigaciones Cientificas y Técnicas, Buenos Aires C1405DJR, Argentina; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom; cLaboratorio de Sistemática y Biología Evolutiva, Museo de La Plata, La Plata B1900FWA, Argentina; dCentro Austral de Investigaciones Científicas, Consejo Nacional de Investigaciones Cientificas y Técnicas, 9410 Ushuaia, Tierra del Fuego, Argentina; and eDepartment of Palaeontology, GNS Science, Lower Hutt 5040, New Zealand Edited by Michael J. Donoghue, Yale University, New Haven, CT, and approved July 15, 2015 (received for review December 10, 2014) The Asteraceae (sunflowers and daisies) are the most diverse Here we report fossil pollen evidence from exposed Campanian/ family of flowering plants. Despite their prominent role in extant Maastrichtian sediments from the Antarctic Peninsula (Fig. 1, Fig. S1, terrestrial ecosystems, the early evolutionary history of this family and SI Materials and Methods, Fossiliferous Localities)(7)thatradi- remains poorly understood. Here we report the discovery of a cally changes our understanding of the early evolution of Asteraceae. number of fossil pollen grains preserved in dinosaur-bearing deposits from the Late Cretaceous of Antarctica that drastically pushes back Results and Discussion the timing of assumed origin of the family. Reliably dated to ∼76–66 The pollen grains reported here and discovered in the Late Cre- Mya, these specimens are about 20 million years older than previ- taceous of Antarctica are tricolporate, microechinate, with long ously known records for the Asteraceae. -

Dry Forest Trees of Madagascar
The Red List of Dry Forest Trees of Madagascar Emily Beech, Malin Rivers, Sylvie Andriambololonera, Faranirina Lantoarisoa, Helene Ralimanana, Solofo Rakotoarisoa, Aro Vonjy Ramarosandratana, Megan Barstow, Katharine Davies, Ryan Hills, Kate Marfleet & Vololoniaina Jeannoda Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK. © 2020 Botanic Gardens Conservation International ISBN-10: 978-1-905164-75-2 ISBN-13: 978-1-905164-75-2 Reproduction of any part of the publication for educational, conservation and other non-profit purposes is authorized without prior permission from the copyright holder, provided that the source is fully acknowledged. Reproduction for resale or other commercial purposes is prohibited without prior written permission from the copyright holder. Recommended citation: Beech, E., Rivers, M., Andriambololonera, S., Lantoarisoa, F., Ralimanana, H., Rakotoarisoa, S., Ramarosandratana, A.V., Barstow, M., Davies, K., Hills, BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) R., Marfleet, K. and Jeannoda, V. (2020). Red List of is the world’s largest plant conservation network, comprising more than Dry Forest Trees of Madagascar. BGCI. Richmond, UK. 500 botanic gardens in over 100 countries, and provides the secretariat to AUTHORS the IUCN/SSC Global Tree Specialist Group. BGCI was established in 1987 Sylvie Andriambololonera and and is a registered charity with offices in the UK, US, China and Kenya. Faranirina Lantoarisoa: Missouri Botanical Garden Madagascar Program Helene Ralimanana and Solofo Rakotoarisoa: Kew Madagascar Conservation Centre Aro Vonjy Ramarosandratana: University of Antananarivo (Plant Biology and Ecology Department) THE IUCN/SSC GLOBAL TREE SPECIALIST GROUP (GTSG) forms part of the Species Survival Commission’s network of over 7,000 Emily Beech, Megan Barstow, Katharine Davies, Ryan Hills, Kate Marfleet and Malin Rivers: BGCI volunteers working to stop the loss of plants, animals and their habitats. -

Indigenous Plant Naming and Experimentation Reveal a Plant–Insect Relationship in New Zealand Forests
Received: 23 February 2020 Revised: 10 August 2020 Accepted: 25 August 2020 DOI: 10.1111/csp2.282 CONTRIBUTED PAPER Indigenous plant naming and experimentation reveal a plant–insect relationship in New Zealand forests Priscilla M. Wehi1,2 | Gretchen Brownstein2 | Mary Morgan-Richards1 1School of Agriculture and Environment, Massey University, Palmerston North, Abstract New Zealand Drawing from both Indigenous and “Western” scientific knowledge offers the 2Manaaki Whenua Landcare Research, opportunity to better incorporate ecological systems knowledge into conserva- Dunedin, New Zealand tion science. Here, we demonstrate a “two-eyed” approach that weaves Indige- Correspondence nous ecological knowledge (IK) with experimental data to provide detailed and Priscilla M. Wehi, Manaaki Whenua comprehensive information about regional plant–insect interactions in Landcare Research, 764 Cumberland New Zealand forests. We first examined Maori names for a common forest Street, Dunedin 9053, New Zealand. Email: [email protected], tree, Carpodetus serratus, that suggest a close species interaction between an [email protected] herbivorous, hole-dwelling insect, and host trees. We detected consistent – Funding information regional variation in both Maori names for C. serratus and the plant insect Foundation for Research, Science and relationship that reflect Hemideina spp. abundances, mediated by the presence Technology; Royal Society of New Zealand of a wood-boring moth species. We found that in regions with moths C. serratus trees are home to more weta than adjacent forest species and that these weta readily ate C. serratus leaves, fruits and seeds. These findings con- firm that a joint IK—experimental approach can stimulate new hypotheses and reveal spatially important ecological patterns. -
Ancistrocladaceae
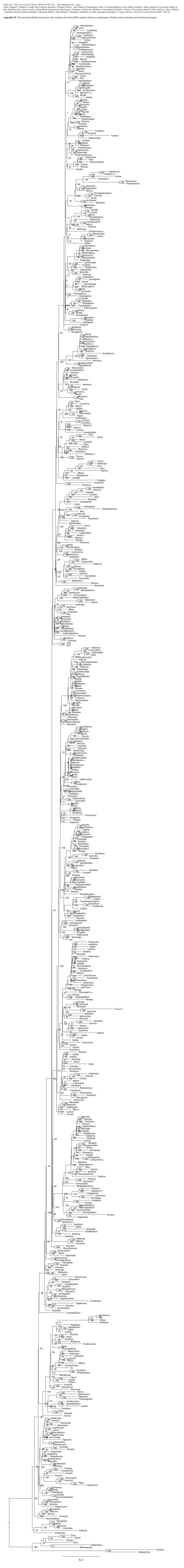
Soltis et al—American Journal of Botany 98(4):704-730. 2011. – Data Supplement S2 – page 1 Soltis, Douglas E., Stephen A. Smith, Nico Cellinese, Kenneth J. Wurdack, David C. Tank, Samuel F. Brockington, Nancy F. Refulio-Rodriguez, Jay B. Walker, Michael J. Moore, Barbara S. Carlsward, Charles D. Bell, Maribeth Latvis, Sunny Crawley, Chelsea Black, Diaga Diouf, Zhenxiang Xi, Catherine A. Rushworth, Matthew A. Gitzendanner, Kenneth J. Sytsma, Yin-Long Qiu, Khidir W. Hilu, Charles C. Davis, Michael J. Sanderson, Reed S. Beaman, Richard G. Olmstead, Walter S. Judd, Michael J. Donoghue, and Pamela S. Soltis. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98(4): 704-730. Appendix S2. The maximum likelihood majority-rule consensus from the 17-gene analysis shown as a phylogram with mtDNA included for Polyosma. Names of the orders and families follow APG III (2009); other names follow Cantino et al. (2007). Numbers above branches are bootstrap percentages. 67 Acalypha Spathiostemon 100 Ricinus 97 100 Dalechampia Lasiocroton 100 100 Conceveiba Homalanthus 96 Hura Euphorbia 88 Pimelodendron 100 Trigonostemon Euphorbiaceae Codiaeum (incl. Peraceae) 100 Croton Hevea Manihot 10083 Moultonianthus Suregada 98 81 Tetrorchidium Omphalea 100 Endospermum Neoscortechinia 100 98 Pera Clutia Pogonophora 99 Cespedesia Sauvagesia 99 Luxemburgia Ochna Ochnaceae 100 100 53 Quiina Touroulia Medusagyne Caryocar Caryocaraceae 100 Chrysobalanus 100 Atuna Chrysobalananaceae 100 100 Licania Hirtella 100 Euphronia Euphroniaceae 100 Dichapetalum 100 -

University of Birmingham How Deep Is the Conflict Between Molecular And
University of Birmingham How deep is the conflict between molecular and fossil evidence on the age of angiosperms? Coiro, Mario; Doyle, James A.; Hilton, Jason DOI: 10.1111/nph.15708 License: None: All rights reserved Document Version Peer reviewed version Citation for published version (Harvard): Coiro, M, Doyle, JA & Hilton, J 2019, 'How deep is the conflict between molecular and fossil evidence on the age of angiosperms?', New Phytologist, vol. 223, no. 1, pp. 83-99. https://doi.org/10.1111/nph.15708 Link to publication on Research at Birmingham portal Publisher Rights Statement: Checked for eligibility 14/01/2019 This is the peer reviewed version of the following article: Coiro, M. , Doyle, J. A. and Hilton, J. (2019), How deep is the conflict between molecular and fossil evidence on the age of angiosperms?. New Phytol. , which has been published in final form at doi:10.1111/nph.15708. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Use of Self-Archived Versions. General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. -

Analysis of Recent Interception Records Reveals Frequent Transport of Arboreal Ants and Potential Predictors for Ant Invasion in Taiwan
insects Article Analysis of Recent Interception Records Reveals Frequent Transport of Arboreal Ants and Potential Predictors for Ant Invasion in Taiwan 1 2 3 4,5,6 7, Ching-Chen Lee , Yi-Ming Weng , Li-Chuan Lai , Andrew V. Suarez , Wen-Jer Wu y , 8, 9,10,11, , Chung-Chi Lin y and Chin-Cheng Scotty Yang * y 1 Center for Ecology and Environment, Department of Life Science, Tunghai University, Taichung 40704, Taiwan; [email protected] 2 Department of Entomology, University of Wisconsin-Madison, Madison, WI 53706, USA; [email protected] 3 Department of Ecological Humanities, Providence University, Taichung 43301, Taiwan; [email protected] 4 Department of Entomology, University of Illinois, Urbana-Champaign, Urbana, IL 61801, USA; [email protected] 5 Department of Evolution, Ecology, and Behavior, University of Illinois, Urbana-Champaign, Urbana, IL 61801, USA 6 Beckman Institute for Science and Technology, Urbana-Champaign, Urbana, IL 61801, USA 7 Department of Entomology, National Taiwan University, Taipei 10617, Taiwan; [email protected] 8 Department of Biology, National Changhua University of Education, Changhua 50007, Taiwan; [email protected] 9 Research Institute for Sustainable Humanosphere, Kyoto University, Kyoto 611-0011, Japan 10 Department of Entomology, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA 11 Department of Entomology, National Chung Hsing University, Taichung 402204, Taiwan * Correspondence: [email protected]; Tel.: +81-774-38-3874 These authors contributed equally to this work. y Received: 22 April 2020; Accepted: 4 June 2020; Published: 8 June 2020 Abstract: We uncovered taxonomic diversity, country of origin and commodity type of intercepted ants at Taiwanese borders based on an 8 year database of 439 interception records. -

Phylogeny and Phylogenetic Nomenclature of the Campanulidae Based on an Expanded Sample of Genes and Taxa
Systematic Botany (2010), 35(2): pp. 425–441 © Copyright 2010 by the American Society of Plant Taxonomists Phylogeny and Phylogenetic Nomenclature of the Campanulidae based on an Expanded Sample of Genes and Taxa David C. Tank 1,2,3 and Michael J. Donoghue 1 1 Peabody Museum of Natural History & Department of Ecology & Evolutionary Biology, Yale University, P. O. Box 208106, New Haven, Connecticut 06520 U. S. A. 2 Department of Forest Resources & Stillinger Herbarium, College of Natural Resources, University of Idaho, P. O. Box 441133, Moscow, Idaho 83844-1133 U. S. A. 3 Author for correspondence ( [email protected] ) Communicating Editor: Javier Francisco-Ortega Abstract— Previous attempts to resolve relationships among the primary lineages of Campanulidae (e.g. Apiales, Asterales, Dipsacales) have mostly been unconvincing, and the placement of a number of smaller groups (e.g. Bruniaceae, Columelliaceae, Escalloniaceae) remains uncertain. Here we build on a recent analysis of an incomplete data set that was assembled from the literature for a set of 50 campanulid taxa. To this data set we first added newly generated DNA sequence data for the same set of genes and taxa. Second, we sequenced three additional cpDNA coding regions (ca. 8,000 bp) for the same set of 50 campanulid taxa. Finally, we assembled the most comprehensive sample of cam- panulid diversity to date, including ca. 17,000 bp of cpDNA for 122 campanulid taxa and five outgroups. Simply filling in missing data in the 50-taxon data set (rendering it 94% complete) resulted in a topology that was similar to earlier studies, but with little additional resolution or confidence. -
Campanulaceae): Review, Phylogenetic and Biogeographic Analyses
PhytoKeys 174: 13–45 (2021) A peer-reviewed open-access journal doi: 10.3897/phytokeys.174.59555 RESEARCH ARTICLE https://phytokeys.pensoft.net Launched to accelerate biodiversity research Systematics of Lobelioideae (Campanulaceae): review, phylogenetic and biogeographic analyses Samuel Paul Kagame1,2,3, Andrew W. Gichira1,3, Ling-Yun Chen1,4, Qing-Feng Wang1,3 1 Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China 2 University of Chinese Academy of Sciences, Beijing 100049, China 3 Sino-Africa Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China 4 State Key Laboratory of Natural Medicines, Jiangsu Key Laboratory of TCM Evaluation and Translational Research, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, China Corresponding author: Ling-Yun Chen ([email protected]); Qing-Feng Wang ([email protected]) Academic editor: C. Morden | Received 12 October 2020 | Accepted 1 February 2021 | Published 5 March 2021 Citation: Kagame SP, Gichira AW, Chen L, Wang Q (2021) Systematics of Lobelioideae (Campanulaceae): review, phylogenetic and biogeographic analyses. PhytoKeys 174: 13–45. https://doi.org/10.3897/phytokeys.174.59555 Abstract Lobelioideae, the largest subfamily within Campanulaceae, includes 33 genera and approximately1200 species. It is characterized by resupinate flowers with zygomorphic corollas and connate anthers and is widely distributed across the world. The systematics of Lobelioideae has been quite challenging over the years, with different scholars postulating varying theories. To outline major progress and highlight the ex- isting systematic problems in Lobelioideae, we conducted a literature review on this subfamily. Addition- ally, we conducted phylogenetic and biogeographic analyses for Lobelioideae using plastids and internal transcribed spacer regions. -

Review of the Sporoderm Ultrastructure of Members of the Asterales S
ISSN 0031-0301, Paleontological Journal, 2006, Vol. 40, Suppl. 5, pp. S656–S663. © Pleiades Publishing, Inc., 2006. Review of the Sporoderm Ultrastructure of Members of the Asterales S. V. Polevova Biological Faculty, Moscow State University, Leninskie gory 1, Moscow, 119992 Russia e-mail: [email protected] Received March 23, 2006 Abstract—Palynomorphological characteristics of the order Asterales are discussed. Particular attention is paid to the pollen morphology of basal families of this group and to that of problematic taxa that are considered as sister groups to the group under study. Ultrastructurally similar sporoderms of several families, including (1) Asteraceae, Calyceraceae, and Goodeniaceae; (2) Campanulaceae, Phellinaceae, and Menyanthaceae; (3) Rousseaceae, Abrophyllaceae, and Columelliaceae, are described. Pollen grains of Alseuosmiaceae and Stylidiaceae show unique ultrastructural features of the exine. DOI: 10.1134/S0031030106110128 Key words: Asterales, pollen grains, ultrastructure, phylogenetic systematics. INTRODUCTION MATERIAL AND METHODS At different times, concepts of the group of Aster- Pollen grains of 18 members of 12 families were aceae and its relatives has been considered to include studied. The material was received from the herbarium different families. These variants concerned a distinct of Komarov Botanical Institution of the Russian Acad- circle of taxa. Thus, the system of Takhatajan (1997) emy of Sciences, St. Petersburg. included the subclass Asteridae with 14 families; the (1) Family Goodeniaceae: Brunonia australis system of Thorne (2000) included the suborder Astera- R. Brown and Dampiera eriocephala Vriese. nae with nine families. (2) Family Columelliaceae: Columellia sericea Recently, relationships of Asteraceae have been sig- F.A. Humbolt, A.J.A. Bonpland et C.S. -

Early Evolution of the Angiosperm Clade Asteraceae in the Cretaceous of Antarctica
Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica Viviana D. Barredaa,1,2, Luis Palazzesia,b,1, Maria C. Telleríac, Eduardo B. Oliverod, J. Ian Rainee, and Félix Forestb aDivisión Paleobotánica, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Consejo Nacional de Investigaciones Cientificas y Técnicas, Buenos Aires C1405DJR, Argentina; bJodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom; cLaboratorio de Sistemática y Biología Evolutiva, Museo de La Plata, La Plata B1900FWA, Argentina; dCentro Austral de Investigaciones Científicas, Consejo Nacional de Investigaciones Cientificas y Técnicas, 9410 Ushuaia, Tierra del Fuego, Argentina; and eDepartment of Palaeontology, GNS Science, Lower Hutt 5040, New Zealand Edited by Michael J. Donoghue, Yale University, New Haven, CT, and approved July 15, 2015 (received for review December 10, 2014) The Asteraceae (sunflowers and daisies) are the most diverse Here we report fossil pollen evidence from exposed Campanian/ family of flowering plants. Despite their prominent role in extant Maastrichtian sediments from the Antarctic Peninsula (Fig. 1, Fig. S1, terrestrial ecosystems, the early evolutionary history of this family and SI Materials and Methods, Fossiliferous Localities)(7)thatradi- remains poorly understood. Here we report the discovery of a cally changes our understanding of the early evolution of Asteraceae. number of fossil pollen grains preserved in dinosaur-bearing deposits from the Late Cretaceous of Antarctica that drastically pushes back Results and Discussion the timing of assumed origin of the family. Reliably dated to ∼76–66 The pollen grains reported here and discovered in the Late Cre- Mya, these specimens are about 20 million years older than previ- taceous of Antarctica are tricolporate, microechinate, with long ously known records for the Asteraceae.