Heavy Metals and Benthic Foraminifera ⇑ Virgínia A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Projeto De Execução De Transposição De
PROJETO DE EXECUÇÃO DE TRANSPOSIÇÃO DE SEDIMENTOS PARA OTIMIZAÇÃO DO EQUILÍBRIO HIDRODINÂMICO NA RIA DE AVEIRO – CANAL DE OVAR ATÉ AO CARREGAL, CANAL DE OVAR ATÉ AO PARDILHÓ E CANAL DA MURTOSA – – CANAL DE ÍLHAVO, CANAIS DO LAGO PARAÍSO E CANAIS DA ZONA CENTRAL DA RIA – RELATÓRIO DE CONFORMIDADE AMBIENTAL DO PROJETO DE EXECUÇÃO VOLUME I – RESUMO NÃO TÉCNICO NOVEMBRO 2017 PROJETO DE EXECUÇÃO DE TRANSPOSIÇÃO DE SEDIMENTOS PARA OTIMIZAÇÃO DO EQUILÍBRIO HIDRODINÂMICO NA RIA DE AVEIRO – CANAL DE OVAR ATÉ AO CARREGAL, CANAL DE OVAR ATÉ AO PARDILHÓ E CANAL DA MURTOSA – – CANAL DE ÍLHAVO, CANAIS DO LAGO PARAÍSO E CANAIS DA ZONA CENTRAL DA RIA – RELATÓRIO DE CONFORMIDADE AMBIENTAL DO PROJETO DE EXECUÇÃO (RECAPE) VOLUME I – RESUMO NÃO TÉCNICO ÍNDICE 1. IDENTIFICAÇÃO DO PROJETO ................................................................................................................ 1 2. ANTECEDENTES DO PROJETO .............................................................................................................. 4 3. DESCRIÇÃO DO PROJETO ...................................................................................................................... 5 3.1 Condições Impostas na DIA para o Projeto de Execução.................................................................. 5 3.2 Solução de Desassoreamento Adotada e Locais de Deposição dos Dragados ................................ 7 3.2.1 Desassoreamento ................................................................................................................... 7 3.2.2 Deposição -

Aveiro Águeda Agadão Agregação Agadão Aveiro Águeda Aguada De Baixo Agregação União Das Freguesias De Barrô E Aguada De Baixo
Reorganização Administrativa do Território das Freguesias - (RATF) Lei n.º 11-A/2013, de 28 de janeiro - Reorganização Administrativa do Território das Freguesias; Declaração de Retificação n.º 19/2013, de 28 de março; Lei n.º 56/2012, de 8 de novembro - Reorganização Administrativa de Lisboa Distrito Concelho Freguesia (JF) Informação adicional Alteração RATF Freguesia criada/alterada pela RATF Informação adicional União das freguesias de Belazaima do Chão, Castanheira do Vouga e Aveiro Águeda Agadão Agregação Agadão Aveiro Águeda Aguada de Baixo Agregação União das freguesias de Barrô e Aguada de Baixo Aveiro Águeda Aguada de Cima Sem alteração Aveiro Águeda Águeda Agregação União das freguesias de Águeda e Borralha Aveiro Águeda Barrô Agregação União das freguesias de Barrô e Aguada de Baixo União das freguesias de Belazaima do Chão, Castanheira do Vouga e Aveiro Águeda Belazaima do Chão Agregação Agadão Aveiro Águeda Borralha Agregação União das freguesias de Águeda e Borralha União das freguesias de Belazaima do Chão, Castanheira do Vouga e Aveiro Águeda Castanheira do Vouga Agregação Agadão Aveiro Águeda Espinhel Agregação União das freguesias de Recardães e Espinhel Aveiro Águeda Fermentelos Sem alteração Aveiro Águeda Lamas do Vouga Agregação União das freguesias de Trofa, Segadães e Lamas do Vouga Aveiro Águeda Macieira de Alcoba Agregação União das freguesias do Préstimo e Macieira de Alcoba Aveiro Águeda Macinhata do Vouga Sem alteração Aveiro Águeda Óis da Ribeira Agregação União das freguesias de Travassô e Óis da Ribeira -

Ria De Aveiro
Road Trips Ria de Aveiro 1 dia é bom, 2 é ótimo, 3 nunca é demais. Road Trips Património Mundial do Centro Beira Baixa Serra da Estrela Médio Tejo Oeste Ria de Ria de Aveiro Região de Coimbra Região de Leiria Aveiro Viseu Dão Lafões 1 dia é bom, 2 é ótimo, 3 nunca é demais. Como usar este roteiro… N 0 E Volta ao Centro de Portugal S Escolha a região que quer conhecer O Centro de Portugal é um território vasto e rico em ex- periências únicas. Esta é apenas uma das propostas que temos para si: uma por cada um dos oito destinos da Re- gião. Mergulhe em cada uma destas regiões, cada uma repleta de história e estórias, descubra o património, as paisagens e muitos segredos bem guardados. Prepare a sua viagem Comece esta aventura mesmo antes de sair de casa, para que nada falhe. Saiba, em cada etapa, onde carregar o Viseu Serra da seu carro elétrico ou avance para as páginas finais deste Ria de Aveiro Dão Estrela roteiro, onde, para além de conselhos úteis, também en- Lafões contrará dicas verdes para uma viagem mais sustentá- vel e amiga do ambiente. Desta forma garantimos uma viagem tão agradável para quem visita o território como para quem o habita. O que pode esperar Região de Ao longo deste roteiro damos-lhe as melhores dicas sobre Coimbra cada local. Esqueça a autoestrada e aventure-se pelo Cen- tro de Portugal por caminhos que são, eles próprios, uma experiência. Descubra os muitos museus, o Património Mundial da Humanidade, as praias mais belas e as mais Região Beira secretas, sem nunca esquecer as iguarias tradicionais de Leiria Baixa nem os melhores locais para captar as fotografias mais instagramáveis. -
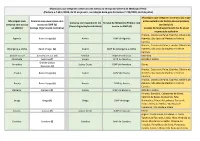
Comarca Com Municípios Com Acesso Ao SMP (B) (Antiga
Municípios que integram comarcas com acesso ao serviço do Sistema de Mediação Penal (Portaria n.º 68-C/2008, de 22 de janeiro, na redação dada pela Portaria n.º 732/2009, de 8 de julho) Municípios que integram a comarca (c) e que Municípios com Comarca com municípios com estão excluídos do âmbito de competência Comarca correspondente (c) Serviço do Ministério Público com comarca com acesso acesso ao SMP (b) territorial do (Nova Organização Judiciária) acesso ao SMP (d) ao SMP(a) (Antiga Organização Judiciária) Sistema de Mediação Penal à luz da atual organização judiciária Arouca, Castelo de Paiva, Espinho, Oliveira de Águeda Baixo Vouga (e) Aveiro DIAP de Águeda Azeméis, São João da Madeira e Vale de Cambra Arouca, Castelo de Paiva, Espinho, Oliveira de Albergaria-a-Velha Baixo Vouga (e) Aveiro DIAP de Albergaria-a-Velha Azeméis, São João da Madeira e Vale de Cambra Alcácer do Sal Alentejo Litoral (e) Setúbal DIAP de Grândola Sesimbra Alcochete Montijo (f) Lisboa DIAP do Montijo Almada e Lisboa Grande Lisboa Amadora Lisboa Oeste DIAP da Amadora Oeiras Noroeste (e) Arouca, Castelo de Paiva, Espinho, Oliveira de Anadia Baixo Vouga (e) Aveiro DIAP de Anadia Azeméis, São João da Madeira e Vale de Cambra Arouca, Castelo de Paiva, Espinho, Oliveira de Aveiro Baixo Vouga (e) Aveiro DIAP de Aveiro Azeméis, São João da Madeira e Vale de Cambra Barreiro Barreiro (f) Lisboa DIAP do Barreiro Almada e Lisboa Amares, Barcelos, Cabeceiras de Basto, Celorico de Basto, Esposende, Fafe, Braga Braga (f) Braga DIAP de Braga Guimarães, Póvoa de Lanhoso, -

1. Enquadramento Territorial
ESTUDOS DE CARACTERIZAÇÃO Capítulo I Enquadramento Territorial Janeiro 2015 REVISÃO DO PDM DE ANADIA Estudos de caracterização e diagnóstico Índice Geral: 1. Enquadramento territorial ................................................................................................................. 1 1.1. Enquadramento Geográfico ....................................................................................................... 1 1.2. Sistema urbano ............................................................................................................................... 3 1.3. Redes Intermunicipais de Infraestruturas ................................................................................... 5 1.3.1. Rede viária nacional/regional ................................................................................................. 5 1.3.2. Infraestruturas Ferroviárias ......................................................................................................... 7 1.3.3. Infraestruturas regionais de energia elétrica e de gás natural ......................................... 7 1.3.4. Tratamento e valorização de resíduos sólidos urbanos ..................................................... 8 Enquadramento Territorial REVISÃO DO PDM DE ANADIA Estudos de caracterização e diagnóstico Índice de Figuras: Figura 1 – Organização administrativa de acordo com a CAOP 2013 ........................................... 1 Figura 2 - Enquadramento geográfico do concelho de Anadia no distrito de Aveiro ............... 2 Figura 3 – Equidistância -

Adenda Ao Contrato De Gestão Entre O Estado
ADENDA AO CONTRATO DE GESTÃO ENTRE O ESTADO PORTUGUÊS, OS MUNICÍPIOS DE ÁGUEDA, ALBERGARIA-A-VELHA, AVEIRO, ESTARREJA, ÍLHAVO, MURTOSA, OLIVEIRA DO BAIRRO, SEVER DO VOUGA E VAGOS E A ADRA – ÁGUA DA REGIÃO DE AVEIRO, S.A. Considerando que, em 23 de Setembro de 2009, foi celebrado entre o Estado, os Municípios de Águeda, Albergaria-a-Velha, Aveiro, Estarreja, Ílhavo, Murtosa, Oliveira do Bairro, Sever do Vouga e Vagos e a ADRA – Águas da Região de Aveiro, S.A. (ADRA), um Contrato de Gestão, por intermédio do qual o Estado e os referidos Municípios atribuíram à ADRA a gestão e a exploração, em regime de exclusivo, do Sistema de Águas da Região de Aveiro ou SARA. Considerando que, nos termos da Cláusula 33.ª do referido Contrato, a modificação substancial do objecto e do âmbito territorial do contrato de gestão só pode operar mediante alteração do Contrato de Parceria nos termos aí contemplados. Considerando que, nos termos da Cláusula 24.ª do Contrato de Parceria, «quando a gestão e a exploração dos serviços de águas relativos ao Sistema passe a abranger partes de sistemas municipais ou sistemas municipais de abastecimento de água para consumo público e de saneamento de águas residuais urbanas não integrados no objecto e no âmbito da Parceria, os municípios interessados celebram com os outorgantes da Parceria uma Adenda à Parceria e competentes anexos, procedendo ainda à delegação prevista na cláusula 2.ª.». Considerando o interesse do Município de Ovar e dos outorgantes do Contrato de Parceria no alargamento do Sistema aos serviços de águas -

6 Caracterização Intermunicipal
6 Caracterização intermunicipal Neste capítulo apresenta-se uma caracterização a nível intermunicipal para a área da AMRia. Para o efeito tem-se em consideração a informação disponibilizada e apresentada no capítulo anterior para cada um dos municípios. Esta síntese consiste numa análise integrada da informação aplicando o modelo DPSIR, com o objectivo de identificar para a AMRia os aspectos mais críticos associados aos recursos hídricos e à sua actual gestão. A informação constante desta síntese será retomada no relatório seguinte e será o ponto de partida para a identificação das linhas orientadoras e estratégias a implementar em cada um dos municípios para uma gestão sustentável do recurso hídrico na área da AMRia. 6.1 Forças motoras 6.1.1 População Relativamente à dinâmica demográfica, verificam-se diferenças entre os concelhos que constituem a AMRia. Aveiro é o concelho com o maior número de habitantes (73 335 habitantes em 2001). Só os concelhos de Aveiro e Ovar englobam 37% (128 533 habitantes) do total da população da AMRia (346 300 habitantes) (Quadro 6.1). Os concelhos com o menor número de habitantes em 2001 são Murtosa (9 458), Mira (12 872) e Sever do Vouga (13 186). Quadro 6.1 – Dados demográficos. Concelhos Área Nº População Variação Densidade (Km2) freguesias residente 2001 população 1991- populacional 2001 (%) (Hab/km2) Centro 28198,7 1334 2348397 3,8 84,3 Águeda 335,3 20 49041 11,3 146,3 Albergaria-a-Velha 155,4 8 24638 12,0 158,5 Aveiro 199,9 14 73335 10,4 366,9 Estarreja 108,4 7 28182 5,4 260,0 Ílhavo 73,5 4 37209 12,0 506,2 Mira 124 4 12872 -2,9 103,8 Murtosa 73,3 4 9458 -1,3 129,0 Oliveira do Bairro 87,3 6 21164 13,4 242,4 Ovar 147,4 8 55198 11,2 374,5 Sever do Vouga 129,6 9 13186 -4,6 101,7 Vagos 164,9 11 22017 15,5 133,5 AMRia 1599 95 346300 9,4 216,6 Fonte: INE, Recenseamento Geral da População e Habitação, 2001. -

Distrito Concelho Freguesia N. Eleitores Mandatos AF Aveiro
N. Mandatos Distrito Concelho Freguesia Eleitores AF Aveiro Águeda Aguada de Cima 3.684 9 Aveiro Águeda Fermentelos 2.827 9 Aveiro Águeda Macinhata do Vouga 3.028 9 Aveiro Águeda União das freguesias de Águeda e Borralha 12.219 13 Aveiro Águeda União das freguesias de Barrô e Aguada de Baixo 3.010 9 Aveiro Águeda União das freguesias de Belazaima do Chão, Castanheira do Vouga e Agadão 1.427 9 Aveiro Águeda União das freguesias de Recardães e Espinhel 5.442 13 Aveiro Águeda União das freguesias de Travassô e Óis da Ribeira 2.033 9 Aveiro Águeda União das freguesias de Trofa, Segadães e Lamas do Vouga 4.091 9 Aveiro Águeda União das freguesias do Préstimo e Macieira de Alcoba 824 7 Aveiro Águeda Valongo do Vouga 4.287 9 Aveiro Albergaria-a-Velha Albergaria-a-Velha e Valmaior 9.647 13 Aveiro Albergaria-a-Velha Alquerubim 2.054 9 Aveiro Albergaria-a-Velha Angeja 1.864 9 Aveiro Albergaria-a-Velha Branca (Albergaria-a-Velha) 4.996 9 Aveiro Albergaria-a-Velha Ribeira de Fráguas 1.547 9 Aveiro Albergaria-a-Velha São João de Loure e Frossos 2.516 9 Aveiro Anadia Avelãs de Caminho 1.139 9 Aveiro Anadia Avelãs de Cima 1.967 9 Aveiro Anadia Moita (Anadia) 2.220 9 Aveiro Anadia Sangalhos 3.734 9 Aveiro Anadia São Lourenço do Bairro 2.278 9 Aveiro Anadia União das freguesias de Amoreira da Gândara, Paredes do Bairro e Ancas 2.621 9 Aveiro Anadia União das freguesias de Arcos e Mogofores 5.714 13 Aveiro Anadia União das freguesias de Tamengos, Aguim e Óis do Bairro 3.068 9 Aveiro Anadia Vila Nova de Monsarros 1.631 9 Aveiro Anadia Vilarinho do Bairro -

Case Study SIMRIA
Case Study Shadow Protect SIMRIA – Saneamento Integrado dos Municípios da Ria, S.A. A SIMRIA – Sistema Multimunicipal de Estrutura e necessidades Saneamento da Ria de Aveiro – As infra-estruturas do Sistema Multimunicipal de adquiriu o ShadowProtect Server e o Saneamento da Ria de Aveiro estendem-se por uma Image Manager Enterprise, duas vasta região geográfica, desde o município de soluções StorageCraft líderes na área Espinho, a norte, até ao de Cantanhede, a sul. O do Disaster Recovery. Sistema Multimunicipal é constituido por um conjunto de 80 estações elevatórias, cinco ETAR’s e ainda Detalhes do Cliente: Com a missão de projecto, construção e exploração quatro centros operacionais: Sede (Aveiro), ETAR » 60 postos e 12 servidores em múltiplos sites » Anel de fibra óptica com WAN a 100Mb do Sistema Multimunicipal de Saneamento da Ria Norte (Cacia), ETAR Sul (Gafanha da Encarnação) e » Exchange 2003 e Windows Server 2003 de Aveiro, numa lógica integrada de requalificação ETAR de Espinho. Todos estes locais encontram-se ambiental e sustentabilidade económica, a SIMRIA interligados por uma infra-estrutura própria de fibra vinha procurando por uma solução com total óptica, com uma velocidade disponibilizada dentro Licenciamento: capacidade para lidar com eventuais cenários de da WAN de 100Mb. No seu conjunto, existem cerca » ShadowProtect Server Edition desastre. Tendo em conta o carácter fundamental de 60 postos, entre desktops e portáteis (com » Image Manager Enterprise com GRE da actividade para a a região, era necessária uma máquinas equipadas com XP, Vista e Windows 7), solução capaz de apresentar total eficácia e bem como 12 servidores aplicacionais e de ficheiros impacto mínimo. -

Accommodation
Accommodation Algarve Albufeira Apartamentos Turisticos Topázio Tourist Apartments / *** Address: Rua Vasco da Gama 8200-908 Albufeira Telephone: +351 289 586 205 Fax: +351 289 586 210 E-mail: [email protected] Website: http://www.detailshotels.com Centro de Portugal Águeda Hotel Estalagem da Pateira Hotel accommodation / Hotel / *** Address: Rua da Pateira, 81 - Fermentelos 3750 - 439 Águeda Telephone: +351 234 721 205 - 234 721 219 Fax: +351 234 722 181 E-mail: [email protected] Website: http://www.pateira.com Anadia Anadia Cabecinho Hotel Hotel accommodation / Hotel / *** Address: Av. Eng. Tavares da Silva 3780-203 Anadia Telephone: +351 231 510 940 Fax: +351 231 510 941 E-mail: [email protected] Website: http://www.hotel-cabecinho.com Aveiro Apartamentos Turísticos Avenida 60 Casa do Pátio Amarelo Tourist Apartments / *** Local accommodation Address: Avenida Lourenço Peixinho, 603800-120 Aveiro Address: Rua Manuel Luís Nogueira, 52 - Rc,3800-214 Telephone: +351 966 909 719; +351 960 468 084; +351 Aveiro 234 840 570 Telephone: +351 933 310 499 E-mail: [email protected] Website: E-mail: [email protected] Website: https://www.innapartments.com/ https://casapatioamarelo.blogspot.pt Home Sweet Home Aveiro Home Sweet Home Aveiro Arrochela Local accommodation Local accommodation Address: Rua Eng Silvério da Silva Pereira3800-175 Address: Rua da Arrochela3810-164 Aveiro Aveiro Telephone: +351 968 020 724 Telephone: +351 968 020 724 E-mail: [email protected] Website: E-mail: [email protected] -

Quintais No Concelho Da Murtosa: Levantamento E Caracterização
Quintais no Concelho da Murtosa: levantamento e caracterização JOSÉ PEDRO DA SILVA TAVARES ARQUITETURA PAISAGISTA DEPARTAMENTO DE GEOCIÊNCIAS, AMBIENTE E ORDENAMENTO DO TERRITÓRIO 2014 Orientador TERESA DULCE PORTELA MARQUES PROFESSORA AUXILIAR FACULDADE DE CIÊNCIAS DA UNIVERSIDADE DO PORTO Todas as correções determinadas pelo júri, e só essas, foram efetuadas. O Presidente do Júri, Porto, ______/______/_________ FCUP i Quintais no Concelho da Murtosa: levantamento e caracterização AGRADECIMENTOS Um Trabalho desta natureza implicou em mim um esforço tremendo (quase) sobrenatural. Foi uma provar de fogo, a que, felizmente fui capaz de responder e ultrapassar… Mais a um desafio ganho! Obviamente que esta vitória teve o grande apoio da família; Pai e Mãe sempre presentes nas birras do benjamim, e as Manas Lola e Sky e Yvette com toda a pachorra do mundo. A todos eles um grande bem-haja. Não esquecendo a paciência e por ter acreditado nesta loucura, a Professora Teresa Marques. Um obrigado do fundo do coração. Para os amigos, sempre inseparáveis, um grande agradecimento. Ao Lito, por todos os dias lembrar-me que vale sempre a pena viver, ao Vítor pelas dicas informáticas, à Maria João e Nuno, os psicólogos de serviço, ao Gil, ao Pedro e Norberto, sem eles, o massacre não teria tanto glamour nem piada, à pandilha da Murtosa. À Cátia e Elvira, as amigas da Bila, sempre preocupadas, ao Luís, sem ti e nem criada malcriada dificilmente chegaria a bom porto… Ao Ricardo, Tiago e Sara pelos seus lembretes “tens de terminar”; Ou ao João, o meu novo patrão, que com suas constantes ameaças de despedimento: sem Mestrado não há salário… Um agradecimento do tamanho do mundo a todos que me ajudaram… FCUP ii Quintais no Concelho da Murtosa: levantamento e caracterização RESUMO Os espaços de produção particulares (nomeadamente os quintais), sempre representaram um papel fundamental no dia-a-dia das populações rurais. -
Plano Director Municipal De Águeda – Revisão
PLANO DIRECTOR MUNICIPAL DE ÁGUEDA – REVISÃO ESTUDOS SECTORIAIS: Infra-estruturas e Transportes Abril 2009 1 PLANO DIRECTOR MUNICIPAL DE ÁGUEDA – REVISÃO NDICE Pág. 1. INTRODUÇÃO 3 2. REDE VIÁRIA E TRANSPORTES – PANORAMA NACIONAL E REGIONAL 4 2.1. PERSPECTIVA NACIONAL 4 2.2 . PERSPECTIVA REGIONAL 13 3. REDE VIÁRIA E TRANSPORTES – PANORAMA MUNICIPAL 20 3.1. ENQUADRAMENTO 20 3.2. REDE VIÁRIA 20 3.2.1. Rede Viária Supra Municipal / Sistema Primário 21 3.2.1.1. Auto-Estrada 25 (A 25) 21 3.2.1.2. Itinerário Complementar 2 (IC2) 23 3.2.1.3. Estrada Nacional n.º 1 (EN 1) 25 3.2.1.4. Estrada Nacional n.º 333 (EN 333) / Variante à EN 333 26 3.2.1.5. Estrada Regional n.º 230 – (antiga EN 230) 27 3.2.1.6. Estrada Regional n.º 336 28 3.2.2. Rede Viária Municipal / Sistema Secundário, Terciário e Quaternário 28 3.2.3. Transportes Colectivos (Rede Rodoviária) 30 3.2.4. Investimentos Municipais em Rede Viária e Transportes 33 3.3. REDE FERROVIÁRIA 34 3.4. INFRA-ESTRUTURA AÉREA 37 3.5. MOVIMENTOS PENDULARES / MOBILIDADE 39 3.6. CONSUMOS ENERGÉTICOS E QUESTÕES AMBIENTAIS 44 GRUPO DE TRABALHO DO PDM ABRIL 2009 2 PLANO DIRECTOR MUNICIPAL DE ÁGUEDA – REVISÃO 4. INFRA-ESTRUTURAS BÁSICAS – PANORAMA NACIONAL E REGIONAL 48 4.1. ABASTECIMENTO DE ÁGUA E SANEAMENTO DE ÁGUAS RESÍDUAIS 48 4.2. REDE DE GÁS (NATURAL) 51 4.3. REDE ELÉCTRICA 53 4.4. REDE DE TELECOMUNICAÇÕES 55 5. INFRA-ESTRUTURAS BÁSICAS – PANORÂMA MUNICIPAL 57 5.1.