PROFILE of Nauclea Diderrichii LEAF EXTRACTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Journal on Taxonomic Botany, Plant Sociology and Ecology
A JOURNAL ON TAXONOMIC BOTANY, LIPI PLANT SOCIOLOGY AND ECOLOGY 12(4) REINWARDTIA A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY Vol. 12(4): 261 - 337, 31 March 2008 Editors ELIZABETH A. WIDJAJA, MIEN A. RIFAI, SOEDARSONO RISWAN, JOHANIS P. MOGEA Correspondece on The Reinwardtia journal and subscriptions should be addressed to HERBARIUM BOGORIENSE, BIDANG BOTANI, PUSAT PENELITIAN BIOLOGI - LIPI, BOGOR, INDONESIA REINWARDTIA Vol 12, Part 4, pp: 285 - 288 NOTES ON MALESIAN NAUCLEEAE Received September 26, 2007; accepted November 5, 2007. C.E.RIDSDALE Nationaal Herbarium Nederland, Universiteit Leiden Branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands. E-mail: [email protected] ABSTRACT RIDSDALE, C.E. 2008. Notes on Malesian Neonaucleea. Reinwardtia 12(4): 285 – 288 — Neonauclea pseudoborneensis, Neonauclea subsessilis and Myrmeconauclea surianii are described as new species. Sarcocephalis fluviatilis Elmer is reinstated as a variety of Myrmeconauclea strigosa. The loss of a large number of type specimens formerly in L is reported. Keyword: Malesia, Neonauclea pseudoborneensis, Neonauclea subsessilis, Myrmeconauclea surianii, Sarcocephalis fluviatilis, Myrmeconauclea strigosa ABSTRAK RIDSDALE, C.E. 2008. Catatan pada Neonaucleea Malesia. Reinwardtia 12(4): 282 – 288 — Neonauclea pseudoborneensis, Neonauclea subsessilis dan Myrmeconauclea surianii diuraikan sebagai jenis baru. Sarcocephalis fluviatilis Elmer direklasifikasi sebagai varietas Myrmeconauclea strigosa. Hilangnya sejumlah tipe specimen yang semula ada di L juga dilaporkan. Kata kunci: Malesia, Neonauclea pseudoborneensis, Neonauclea subsessilis, Myrmeconauclea surianii, Sarco- cephalis fluviatilis, Myrmeconauclea strigosa NEONAUCLEA conicis ochraceis papillatis. Corolla infundibularis 11- 14 mm longa, glabra, lobis ovatis. Stylus per 8-12 mm Since the published revisions of Naucleeae exsertus. Capitula fructifera 30-35 mm diam., fructibus 8 (Ridsdale 1978) and Neonauclea (Ridsdale 1989) mm longis. -

Neonauclea Glabra Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Neonauclea glabra Click on images to enlarge Family Rubiaceae Scientific Name Neonauclea glabra (Roxb.) Bakh.f. & Ridsdale Ridsdale, C.E. (1989) Blumea 34: 240. Common name Flowers. Copyright CSIRO Maple, Yellow; Yellow Maple; Moody Gum; Hard Leichhardt; Burr Tree; Yellow Hickory Stem Fresh blaze almost yellow but turning greenish brown on exposure. Leaves Stipules spathulate, broader and rounded towards the apex. Pairs of stipules enclosing the terminal bud. Leaf blades about 7-20 x 4.5-9 cm. Foveoles, if present, small and inconspicuous, opening in a narrow slit. Flowers Leaves and Flowers. Copyright CSIRO Flowers attached by their bases to a receptacle to form a perfectly spherical head of flowers. Corolla tube about 4-5 mm long, much longer than the lobes, corolla lobes about 1-1.5 mm long. Anthers shortly stalked, attached below the apex of the corolla tube, anthers not or only very slightly exserted. Ovules numerous on pendulous placentas. Fruit Fruits spherical, about 15 mm diam. Seeds numerous in each aggregate fruit. Seeds winged at each end, seed + wings about 3 x 0.5 mm. Embryo about 0.9-1 x 0.5 mm. Seedlings Scale bar 10mm. Copyright CSIRO Cotyledons elliptic, about 1-2 mm long, hairy on the upper surface. At the tenth leaf stage: leaf blade narrowly elliptic, apex acute, base cuneate, glabrous, midrib flush with the upper surface of the leaf blade; stipules narrowly triangular, apex acute. -

Complete Index of Common Names: Supplement to Tropical Timbers of the World (AH 607)
Complete Index of Common Names: Supplement to Tropical Timbers of the World (AH 607) by Nancy Ross Preface Since it was published in 1984, Tropical Timbers of the World has proven to be an extremely valuable reference to the properties and uses of tropical woods. It has been particularly valuable for the selection of species for specific products and as a reference for properties information that is important to effective pro- cessing and utilization of several hundred of the most commercially important tropical wood timbers. If a user of the book has only a common or trade name for a species and wishes to know its properties, the user must use the index of common names beginning on page 451. However, most tropical timbers have numerous common or trade names, depending upon the major region or local area of growth; furthermore, different species may be know by the same common name. Herein lies a minor weakness in Tropical Timbers of the World. The index generally contains only the one or two most frequently used common or trade names. If the common name known to the user is not one of those listed in the index, finding the species in the text is impossible other than by searching the book page by page. This process is too laborious to be practical because some species have 20 or more common names. This supplement provides a complete index of common or trade names. This index will prevent a user from erroneously concluding that the book does not contain a specific species because the common name known to the user does not happen to be in the existing index. -

Terminalia Ivorensis A.Chev and Nauclea Diderrichii De Wild
An International Multi-Disciplinary Journal, Ethiopia Vol. 3 (4), July, 2009 ISSN 1994-9057 (Print) ISSN 2070-0083 (Online) Fusarium Damping-off of two Timber Species (Terminalia Ivorensis A. Chev and Nauclea Diderrichii De Wild and Th.Dur) in the Nursery (Pp. 252-260) Omokhua, G. E. - Department of Forestry and Wildlife Management, Faculty of Agriculture, University of Port Harcourt, PMB 5323, Port Harcourt, Rivers State, Nigeria E-mail: [email protected] Godwin-Egein, M. I. - Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, PMB 5323, Port Harcourt, Rivers State. Nigeria. Okereke, V. C. - Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, PMB 5323, Port Harcourt, Rivers State. Nigeria. E-mail: [email protected] Abstract The incidence of the damping–off disease of two timber species Terminalia ivorensis and Nauclea diderrichii sown in ground granite, sharp river sand, topsoil and sawdust was assessed at the nursery site of the Department of Forestry and Wildlife Management, University of Port Harcourt. The experiment was laid out in a completely randomised design replicated three times. Fusarium oxysporum was implicated as the causal agent of the disease. Terminalia ivorensis was not susceptible to Fusarium damping-off in the study. A significant effect (P<0.05) was observed in top soil which recorded the highest disease incidence in Nauclea diderrichii. Saw dust showed 0% disease incidence and supported the highest plant growth in both Copyright © IAARR, 2009: www.afrrevjo.com 252 Indexed African Journals Online: www.ajol.info Fusarium Damping-off of two Timber Species… in the Nursery species. -

African Map Index July 29, 2014
[Type text] African Map Index July 29, 2014 COUNTRY COMMON NAME BOTANICAL NAME Size Req’d (inches) HxWx2.5” Algeria Algerian Oak Quercus canariensis 6 x 6 Angola Black Limba Terminalia superba 4 x 4 Benin Jakkalsbessie Diospyros mespilitormis 2 x 1 Botswana Sausage Tree Kigelia africana 3 x 3 Burkina Faso Zebrano Microberlinia brazzavillensis 3 x 3 Burundi African Olive Olea capensis 1 x 1 Cameroon Black Frake Terminalia superba 4 x 3 Central African Republic Tamarind Tamarindus indica 3 x 4 Chad Carob Ceratonia siliqua 5 x 4 Congo Ebony Diospyros crassiflora 3 x 3 Cote D’Ivoire Makore Tieghemella heckelii 2 x 2 Dem Republic of Congo Bubinga Guibourtia demeusei 7 x 6 Djibouti African Juniper Juniperus procera 1 x 1 Egypt Phoenician Juniper Juniperus phoenicea 3 x 3 Equatorial Guinea Ekuone Coelocaryon preussii 1 x 1 Eritrea Marula Sclerocarya birrea 3 x 1 Ethiopia Opepe Nauclea diderrichii 5 x 5 Gabon Avodire Turraeanthus africanus 2 x 2 Gambia Doussie Afzelia bipindensis 2 x 1 Ghana Afrormosia Pericopsis elata 2 x 2 Guinea Movingui Distemonanthus benthamianus 2 x 3 Guinea-Bissau Flatcrown Albizia adainthifolia 1 x 1 Kenya Yellowwood Podocarpus latifolius 3 x 3 Liberia Thin Winn Millettia leucantha 2 x 2 Libya European Olive Olea europaea 4 x 5 Madagascar Madagascar Rosewood Dalbergia baronii 5 x 2 Malawi Moepel Mimusops caffra 3 x 1 Mali African Walnut Plukenetia conophora 5 x 5 [Type text] African Map Index July 29, 2014 Mauritania African Afzelia Afzelia africana 4 x 4 Morocco Thuja Burl Tetraclinis articulata 3 x 4 Mozambique Wenge -

Dimensional Stability of Nine Tropical Hardwoods from Cameroon
Journal of Tropical Forest Science 22(4): 389–396 (2010) Shukla SR & Kamdem DP DIMENSIONAL STABILITY OF NINE TROPICAL HARDWOODS FROM CAMEROON SR Shukla* & DP Kamdem** Laboratory of Wood Science and Technology, Department of Forestry, 126 Natural Resources Building, Michigan State University, East Lansing MI 48824, USA Received September 2009 SHUKLA SR & KAMDEM DP. 2010. Dimensional stability of nine tropical hardwoods from Cameroon. This study investigated the rate of swelling and dimensional stability of nine tropical hardwood species from Cameroon, namely, ayous (Triplochiton scleroxylon), bilinga (Nauclea diderrichii), bubinga (Guibourtia tessmannii), iroko (Chlorophora excelsa), makore (Mimusops heckelii), moabi (Baillonella toxisperma), movingui (Distemonanthus benthamianus), teak (Tectona grandis) and zingana (Microberlinia brazzavillensis). Continuous swelling of wood specimens immersed in water at room temperature for up to a maximum of 48 hours were monitored using linear voltage displacement transducers (LVDTs). The amount of water uptake as function of immersion duration was measured and correlated with wood porosity. Among the species used in this study, teak showed the lowest swelling rate, therefore, the more dimensionally stable property. Ayous, iroko and movingui were relatively more dimensionally stable than bubinga, makore and moabi. The swelling rate in the tangential direction was much higher than the radial. Bubinga, bilinga and zingana exhibited higher radial swelling rates compared with iroko, teak and makore. Similarly, higher tangential swelling rates were obtained for bubinga and movingui in comparison with teak, makore and moabi. The calculated ratios of the tangential to radial swelling rates also known as the anisotropy of the species used in this study were within the normal range and in agreement with published data. -

Short Communication Tree Density and Growth Rate of Nauclea Diderrichii and Terminalia Ivorensis in the Arboretum of Forestry
Journal of Agriculture and Food Environment Volume 6(2): 1-5, 2019 Onilude, 2019 Short Communication Tree Density and Growth Rate of Nauclea diderrichii and Terminalia ivorensis in the Arboretum of Forestry Research Institute of Nigeria Onilude, Q.A. Forestry Research Institute of Nigeria, P.M.B 5054, Jericho Hill, Ibadan, Nigeria E-mails: [email protected]; [email protected] Received 28th April, 2019; Accepted 6th June, 2019; Corrected 30th June, 2019 Abstract Stands of Nauclea diderrichii and Terminalia ivorensis within the arboretum of Forestry Research Institute of Nigeria were assessed for stand density and diameter growth rates of the tree species. Total enumeration of all the trees for both species was carried out and their diameters at breast height (dbh) measured. Nauclea diderrichii stand had the highest density of 430 trees ha-1 and a total basal area of 4.32m2ha-1 while Terminalia ivorensis stand had a density of 400 trees ha-1 and a total basal area of 26.83m2ha-1. The diameter growth rates for the two species were determined by calculating their mean annual increments (MAIs) in DBH. Nauclea diderrichii had an average DBH of 10.98cm and a mean annual increment (MAI) of 1.10cmyr-1 while Terminalia ivorensis had an average DBH of 24.31cm and MAI of 2.43cmyr-1. This study showed that Mean annual increment generally increased with reduction in stand density. Permanent sample plots (PSP) should be established in the arboretum for continuous inventory to provide data for academic and research activities; and also for sustainable management of the tree resources. -

A Dictionary of the Plant Names of the Philippine Islands," by Elmer D
4r^ ^\1 J- 1903.—No. 8. DEPARTMEl^T OF THE IE"TEIlIOIi BUREAU OF GOVERNMENT LABORATORIES. A DICTIONARY OF THE PLAIT NAMES PHILIPPINE ISLANDS. By ELMER D, MERRILL, BOTANIST. MANILA: BUREAU OP rUKLIC I'RIN'TING. 8966 1903. 1903.—No. 8. DEPARTMEE^T OF THE USTTERIOR. BUREAU OF GOVEENMENT LABOEATOEIES. r.RARV QaRDON A DICTIONARY OF THE PLANT PHILIPPINE ISLANDS. By ELMER D. MERRILL, BOTANIST. MANILA: BUREAU OF PUBLIC PRINTING. 1903. LETTEE OF TEANSMITTAL. Department of the Interior, Bureau of Government Laboratories, Office of the Superintendent of Laboratories, Manila, P. I. , September 22, 1903. Sir: I have the honor to submit herewith manuscript of a paper entitled "A dictionary of the plant names of the Philippine Islands," by Elmer D. Merrill, Botanist. I am, very respectfully. Paul C. Freer, Superintendent of Government Laboratories. Hon. James F. Smith, Acting Secretary of the Interior, Manila, P. I. 3 A DICTIONARY OF THE NATIVE PUNT NAMES OF THE PHILIPPINE ISLANDS. By Elmer D. ^Ikkrii.i., Botanist. INTRODUCTIOX. The preparation of the present work was undertaken at the request of Capt. G. P. Ahern, Chief of the Forestry Bureau, the objeet being to facihtate the work of the various employees of that Bureau in identifying the tree species of economic importance found in the Arcliipelago. For the interests of the Forestry Bureau the names of the va- rious tree species only are of importance, but in compiling this list all plant names avaliable have been included in order to make the present Avork more generally useful to those Americans resident in the Archipelago who are interested in the vegetation about them. -

BILINGA Page 1Of 4
BILINGA Page 1of 4 Family: RUBIACEAE (angiosperm) Scientific name(s): Nauclea diderrichii Sarcocephalus spp. (synonymous) Nauclea gilletii Commercial restriction: no commercial restriction WOOD DESCRIPTION LOG DESCRIPTION Color: orange - yellow Diameter: from 60 to 90 cm Sapwood: clearly demarcated Thickness of sapwood: from 3 to 5 cm Texture: medium Floats: no Grain: interlocked Log durability: good Interlocked grain: marked Note: Heartwood golden yellow or orangey yellow slightly moiré. In interior end-uses, the color remains stable. PHYSICAL PROPERTIES MECHANICAL AND ACOUSTIC PROPERTIES Physical and mechanical properties are based on mature heartwood specimens. These properties can vary greatly depending on origin and growth conditions. Mean Std dev. Mean Std dev. Specific gravity *: 0,76 0,07 Crushing strength *: 63 MPa 7 MPa Monnin hardness *: 5,3 1,3 Static bending strength *: 95 MPa 11 MPa Coeff. of volumetric shrinkage: 0,55 % 0,05 % Modulus of elasticity *: 14660 MPa 1934 MPa Total tangential shrinkage (TS): 7,5 % 0,9 % Total radial shrinkage (RS): 4,5 % 0,7 % (*: at 12% moisture content, with 1 MPa = 1 N/mm²) TS/RS ratio: 1,7 Fiber saturation point: 25 % Musical quality factor: 111,3 measured at 2492 Hz Stability: moderately stable to stable NATURAL DURABILITY AND TREATABILITY Fungi and termite resistance refers to end-uses under temperate climate. Except for special comments on sapwood, natural durability is based on mature heartwood. Sapwood must always be considered as non-durable against wood degrading agents. E.N. = Euro Norm Funghi (according to E.N. standards): class 1 - very durable Dry wood borers: durable - sapwood demarcated (risk limited to sapwood) Termites (according to E.N. -

On the Flora of Australia
L'IBRARY'OF THE GRAY HERBARIUM HARVARD UNIVERSITY. BOUGHT. THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEING AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. r^/f'ORElGN&ENGLISH' <^ . 1859. i^\BOOKSELLERS^.- PR 2G 1.912 Gray Herbarium Harvard University ON THE FLORA OF AUSTRALIA ITS ORIGIN, AFFINITIES, AND DISTRIBUTION. I I / ON THE FLORA OF AUSTRALIA, ITS ORIGIN, AFFINITIES, AND DISTRIBUTION; BEIKG AN TO THE FLORA OF TASMANIA. BY JOSEPH DALTON HOOKER, M.D., F.R.S., L.S., & G.S.; LATE BOTANIST TO THE ANTARCTIC EXPEDITION. Reprinted from the JJotany of the Antarctic Expedition, Part III., Flora of Tasmania, Vol. I. LONDON : LOVELL REEVE, HENRIETTA STREET, COVENT GARDEN. 1859. PRINTED BY JOHN EDWARD TAYLOR, LITTLE QUEEN STREET, LINCOLN'S INN FIELDS. CONTENTS OF THE INTRODUCTORY ESSAY. § i. Preliminary Remarks. PAGE Sources of Information, published and unpublished, materials, collections, etc i Object of arranging them to discuss the Origin, Peculiarities, and Distribution of the Vegetation of Australia, and to regard them in relation to the views of Darwin and others, on the Creation of Species .... iii^ § 2. On the General Phenomena of Variation in the Vegetable Kingdom. All plants more or less variable ; rate, extent, and nature of variability ; differences of amount and degree in different natural groups of plants v Parallelism of features of variability in different groups of individuals (varieties, species, genera, etc.), and in wild and cultivated plants vii Variation a centrifugal force ; the tendency in the progeny of varieties being to depart further from their original types, not to revert to them viii Effects of cross-impregnation and hybridization ultimately favourable to permanence of specific character x Darwin's Theory of Natural Selection ; — its effects on variable organisms under varying conditions is to give a temporary stability to races, species, genera, etc xi § 3. -

High Frequency Regeneration of Plants Via Callus-Mediated Organogenesis
www.nature.com/scientificreports OPEN High frequency regeneration of plants via callus-mediated organogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia Cadamba) Hao Huang1, Ying Wei1, Yongjin Zhai1, Kunxi Ouyang2, Xiaoyang Chen2* & Longhua Bai1* In this works, a simple, efcient and repeatable protocol was developed for in vitro regeneration via callus-mediated organogenesis of Neolamarkia Cadamba using cotyledonary petioles and hypocotyls. Efects of basal medium, plant growth regulators, the types and age of explant on the formation of adventitious buds/shoots were studied. Meanwhile, histological analysis for early ontogenic stages and genetic stability assessment by fow cytometry were investigated. Our investigation demonstrated that, compared with 6-benzyladenine (BA), N6-(2-isopentenyl) adenine (2-ip), Thidiazuron (TDZ) was the optimal cytokinin for buds/shoots induction on cotyledon and hypocotyl explants. Douglas-fr and sugar pine medium (DCR) supplemented with 22.7 μM TDZ and 0.27 μM α-naphthalene acetic acid (NAA) was most efective on bud induction, with the highest bud-induction rate and numbers of buds on cotyledon and hypocotyl explants. The available shoot per explant hit 35.2 when the induced callus sub-cultured to a medium without TDZ. It was found that TDZ could promote induction of the callus and the buds, however, continuous exposure beyond 4 weeks of supplemented high concentration (exceed 11.35 μM), TDZ was harmful to the proliferation and growth of buds/shoots. DCR appeared more efciency than Murashige and Skoog medium (MS), Woody Plant medium (WPM), anther culture of cereal crops medium (N6) on bud induction. Age of cotyledon and hypocotyl explants in 20-day to 25- day was most benefcial to adventitious buds/shoots formation. -
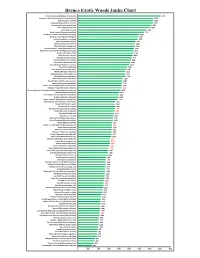
Janka Hardness Chart
Brenco Exotic Woods Janka Chart African Blackwood (Dalbergia melanoxylon) 4050 Kampanga / Snake Bean (Swartzia madagascariensis) 3690 Ipe (Tabebula serratifolia) 3680 Leadwood (Krugiodendron ferreum) 3660 Cumarurana (Taralea oppositifolia) 3590 Cumaru (Dipteryx odorata) 3540 Azobe (Lophira alata) 3350 Ebony Gaboon (Diospyros crassiflora) 3220 Rhodesian / Zambezi Teak (Baikiaea plurijuga) 2990 Pau Rosa / Dina (Swartzia fistuloides) 2940 Tali (Erythrophleum suaveolens) 2920 Jatoba (Hymenaea courbaril) 2820 Afina (Strombosia glaucescens) 2780 Okan (Cylicodiscus gabunensis) 2780 Pearwood African - Aboga (Mimusops lacera) 2730 Purpleheart South American (Peltogyne paniculata) 2710 Sucupira (Bowdichia nitida) 2700 Niove (Staudtia stipitata) 2680 Apomé (Cynometra ananta) 2630 Ataa (Pentaclethra macrophylla) 2580 Eveuss (Klainedoxa gabonensis) 2550 Missanda (Erythrophleum africanum) 2513 Difou (Morus mesozygia) 2400 Greenheart (Chlorocardium rodiei) 2350 Mufuka (Marquesia macroura) 2318 Mukulungu (Austanella congolensis) 2310 Mesquite (Prosopis glandulosa) 2340 Muhuhu (Brachylaena hutchinsii) 2190 Essia (Combretodendron macrocarpum) 2180 Tigerwood (Astronium graveolens) 2160 Danta - Akumaba (Nesogordonia papaverifera) 2140 Sucupira - Tatabu (Diplotropis purpurea) 2140 African Rosewood / Copalwood Rhodesian (Guibourtia coleosperma) 2090 Chanfuta (Afzelia quanzensis) 2020 Vessambata / African Oak (Oldfieldia africana) 1990 Bubinga (Guibourtia demeusei) 1980 Danta - Kamema (Nesogordonia kabingaensis) 1940 Pecan (Carya illinoensis) Pecan Hickory