The Geochemistry of Modern Calcareous Barnacle Shells and Applications for Palaeoenvironmental Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
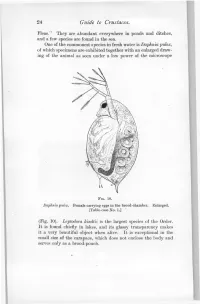
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

A New Species of Lepas (Crustacea: Cirripedia: Pedunculata) from the Miocene Mizunami Group, Japan
Bulletin of the Mizunami Fossil Museum, no. 31 (2004), p. 91-93, 1 fig. c 2004, Mizunami Fossil Museum A new species of Lepas (Crustacea: Cirripedia: Pedunculata) from the Miocene Mizunami Group, Japan Hiroaki Karasawa*, Toshio Tanaka* *, and Yoshitsugu Okumura*** * Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan <[email protected]> ** Aichi Gakuin Junior College, Chikusa, Nagoya 464-8650, Japan < [email protected]> *** Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan <[email protected]> Abstract Lepas kuwayamai, a new species of a lepadid thracican is described from the lower Miocene Mizunami Group in Gifu Prefecture, Honshu, Japan. This represents the third record for the family from Miocene deposits of Japan. Key words: Crustacea, Cirripedia, Thoracica, Pedunculata, Lepadidae, Lepas, Miocene, Japan Material examined: MFM9043, holotype; 8 paratypes, Introduction MFM9044-9051; Coll. M. Kuwayama in, 2004. All specimens are housed in the Mizunami Fossil Museum. The lepadomorph family Lepadidae Darwin, 1851, is a Diagnosis: Lepas with moderate sized capitulum. Shell small group including six genera (Newman, 1996). Among thick. Scutum triangular, slightly higher than wide, with these, the genus Lepas Linnaeus, 1758, is only known clear growth lines; umbonal tooth absent; very weak from Japan in the fossil record. O’hara et al. (1976) reported radial striae sometimes present; apicoumbonal ridge Lepas sp. from the middle Pleistocene Shimosa Group and weak. Tergum flattened without radial striae; occludent unnamed Miocene species was reported from the lower margin convex, rounded. Carina broad. Miocene Morozaki Group (Mizuno and Takeda, 1993) and Etymology: In honor to our friend, Mr. -

Title FEEDING PREFERENCES and RATES of the SNAIL, IANTHINA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository FEEDING PREFERENCES AND RATES OF THE SNAIL, IANTHINA PROLONGATA, THE BARNACLE, LEPAS Title ANSERIFERA, THE NUDIBRANCHS, GLAUCUS ATLANTICUS AND FIONA PINNATA, AND THE FOOD WEB IN THE MARINE NEUSTON Author(s) Bieri, Robert PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1966), 14(2): 161-170 Issue Date 1966-06-30 URL http://hdl.handle.net/2433/175429 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University FEEDING PREFERENCES AND RATES OF THE SNAIL, IANTHINA PROLONGATA, THE BARNACLE, LEPAS ANSERIFERA, THE NUDIBRANCHS, GLAUCUS ATLANTICUS AND FIONA PINNATA, AND THE FOOD WEB IN THE MARINE NEUSTON'l RoBERT BIERI Antioch College, Yellow Springs, Ohio With Plates III-IV and 1 Text-figure On the afternoon of November 5, 1965 during strong north-northwest winds, Porpita, Velella, Physalia, G!aucus, Lepas, Fiona, and three species of Ianthina were blown onto the north beach of the Seto Marine Biological Laboratory. In the Laboratory with an ample supply of running sea water, many of these animals lived for several days and I was able to observe their feeding preferences and rates. On the basis of these obervations and the reports of other workers it is possible to construct a preliminary diagram of the neuston food web. Ianthina prolongata Of the three species of Ianthina washed ashore, I. janthina forma balteata, I. umbilicata, and I. prolongata, I. prolongata was most abundant. I was able to collect more than 160 of them in less than an hour. -

UNIVERSITY of CALIFORNIA, SAN DIEGO Abundance and Ecological
UNIVERSITY OF CALIFORNIA, SAN DIEGO Abundance and ecological implications of microplastic debris in the North Pacific Subtropical Gyre A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Oceanography by Miriam Chanita Goldstein Committee in charge: Professor Mark D. Ohman, Chair Professor Lihini I. Aluwihare Professor Brian Goldfarb Professor Michael R. Landry Professor James J. Leichter 2012 Copyright Miriam Chanita Goldstein, 2012 All rights reserved. SIGNATURE PAGE The Dissertation of Miriam Chanita Goldstein is approved, and it is acceptable in quality and form for publication on microfilm and electronically: PAGE _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ Chair University of California, San Diego 2012 iii DEDICATION For my mother, who took me to the tidepools and didn’t mind my pet earthworms. iv TABLE OF CONTENTS SIGNATURE PAGE ................................................................................................... iii DEDICATION ............................................................................................................. iv TABLE OF CONTENTS ............................................................................................. v LIST OF FIGURES -

The Cirripedia of New Caledonia
The Cirripedia of New Caledonia Diana S. lONES Western Australian MlISeum [email protected] The Indo-Pacific deep-sea benthos was investigated by major expeditions such as those of «Challenger» (1873-1876), «Investigator» (1884-] 887), «Valdiva» (1898-] 899), «Siboga» (1899 1900), «Albatross» (1907-1910) and «Galathea» (1950-52). However, none of these expeditions col lected in the waters of New Caledonia and its surrounding areas. The cirripede fauna of the region was first documented through the brief report of Fischer (1884), who described the shallow water bar nacles of New Caledonia. Fischer briefly listed 15 species from specimens deposited in the Musee de Bordeaux by the missionaries Montrouzier and Lambert. From that time, there was no documenta tion of the fauna until the latter half of the 20th century, when a rigorous collection and taxonomic program was conducted in the region supported through IRD (ORSTOM) and the Museum national d'Histoire naturelle, Paris. Since 1978, numerous barnacle specimens have been collected in the deep waters off Vanuatu (MUSORSTOM 8 1994), New Caledonia, the Chesterfield and Loyalty Islands (BIOCAL 1985, MUSORSTOM 41985, LAGON 1985, MUSORSTOM 5 1986.CHALCAL2 1986, SMIB21986.SMIB31987.CORAIL2 1988,MUSORSTOM61989.VAUBAN 1989,ALIS 1989, SMIB61990,BERYX21992,BATHUS21993,SMIB81993,HALIPR0219(6),the Wall ace and Futuna Islands, Combe. Field. Tuscarora and Waterwich Banks (MUSORSTOM 7 1(92). the Norfolk Ridge (SMIB 4 1989, SMIB 5 1989. BATHUS 3 1993, BATHUS 4 19(4) and the Matthew and Hunter Islands (VOLSMAR 1989). Examination of these collections has yielded an exceptional diversity of thoracican cirripedes. Buckeridge (1994, 1997) provided a comprehensive account of the deep-sea Verrucomorpha (Cirripedia) from collections made by several French cruises in the New Caledonian area and the Wallis and Futuna Islands. -

Download PDF File
Scientific Note Record of coastal colonization of the Lepadid goose barnacle Lepas anatifera Linnaeus, 1758 (Crustacea: Cirripedia) at Arraial do Cabo, RJ 1 1,2 LUIS FELIPE SKINNER & DANIELLE FERNANDES BARBOZA 1Universidade do Estado do Rio de Janeiro (UERJ), Laboratório de Ecologia e Dinâmica Bêntica Marinha, São Gonçalo, Rio de Janeiro. Rua Francisco Portela 1470, Patronato, São Gonçalo, RJ. 24435-005. 2Bolsista PROATEC, UERJ. E-mail: [email protected] Abstract. In this note we record the occurence of Lepas anatifera (Cirripedia) on the hull of a coastal supply boat that operates only in restricted inshore waters of Arraial do Cabo. This species is usually found on tropical and subtropical oceanic waters and, at region, could be classified as transient species. Key words: marine fouling, barnacle, recruitment, inshore transport, species distribution, Cabo Frio upwelling Resumo. Registro de colonização costeira da craca pedunculada Lepas anatifera Linnaeus, 1758 (Crustacea: Cirripedia) na costa de Arraial do Cabo, RJ. Nesta nota nós registramos a ocorrência de Lepas anatifera (Cirripedia) no casco de uma pequena embarcação que opera exclusivamente em águas costeiras de Arraial do Cabo. Esta espécie é típica de águas oceânicas de regiões tropicais e subtropicais e pode ser classificada como transiente na região. Palavras chave: incrustação marinha, craca, recrutamento, transporte costeiro, distribuição de espécies, ressurgência de Cabo Frio Lepas anatifera Linnaeus, 1758 (Fig. 1) is a and not by larval preference. pedunculate goose barnacle with cosmopolitan At Cabo Frio region is frequent to find many distribution (Young 1990, 1998). They are found individuals that arrived to the coast on floating mainly on oceanic waters from tropical and debris. -

University of Cape Town
The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Taxonomy, Systematics and Biogeography of South African Cirripedia (Thoracica) Aiden Biccard Town A thesis submitted in fulfilment of the degreeCape of Master of Science in the Department of Zoology, Faculty of Science, University of Cape Town Supervisor Prof. Charles L. Griffiths University 1 Town “and whatever the man called every livingCape creature, that was its name.” - Genesis 2:19 of University 2 Plagiarism declaration This dissertation documents the results of original research carried out at the Marine Biology Research Centre, Zoology Department, University of Cape Town. This work has not been submitted for a degree at any other university and any assistance I received is fully acknowledged. The following paper is included in Appendix B for consideration by the examiner. As a supervisor of the project undertaken by T. O. Whitehead, I participated in all of the field work and laboratory work involved for the identification of specimens and played a role in the conceptualisation of the project. Figure 1 was compiled by me. Town Whitehead, T. O., Biccard, A. and Griffiths, C. L., 2011. South African pelagic goose barnacles (Cirripedia, Thoracica): substratum preferences and influences of plastic debris on abundance and distribution. Crustaceana, 84(5-6): 635-649. -

Testing Adaptive Hypotheses on the Evolution of Larval Life History in Acorn and Stalked Barnacles
Received: 10 May 2019 | Revised: 10 August 2019 | Accepted: 19 August 2019 DOI: 10.1002/ece3.5645 ORIGINAL RESEARCH Testing adaptive hypotheses on the evolution of larval life history in acorn and stalked barnacles Christine Ewers‐Saucedo1 | Paula Pappalardo2 1Department of Genetics, University of Georgia, Athens, GA, USA Abstract 2Odum School of Ecology, University of Despite strong selective pressure to optimize larval life history in marine environ‐ Georgia, Athens, GA, USA ments, there is a wide diversity with regard to developmental mode, size, and time Correspondence larvae spend in the plankton. In the present study, we assessed if adaptive hypoth‐ Christine Ewers‐Saucedo, Zoological eses explain the distribution of the larval life history of thoracican barnacles within a Museum of the Christian‐Albrechts University Kiel, Hegewischstrasse 3, 24105 strict phylogenetic framework. We collected environmental and larval trait data for Kiel, Germany. 170 species from the literature, and utilized a complete thoracican synthesis tree to Email: ewers‐[email protected]‐kiel. de account for phylogenetic nonindependence. In accordance with Thorson's rule, the fraction of species with planktonic‐feeding larvae declined with water depth and in‐ creased with water temperature, while the fraction of brooding species exhibited the reverse pattern. Species with planktonic‐nonfeeding larvae were overall rare, follow‐ ing no apparent trend. In agreement with the “size advantage” hypothesis proposed by Strathmann in 1977, egg and larval size were closely correlated. Settlement‐com‐ petent cypris larvae were larger in cold water, indicative of advantages for large ju‐ veniles when growth is slowed. Planktonic larval duration, on the other hand, was uncorrelated to environmental variables. -

Planktonic Associations Between Medusae (Classes Scyphozoa and Hydrozoa) and Epifaunal Crustaceans
Planktonic associations between medusae (classes Scyphozoa and Hydrozoa) and epifaunal crustaceans Kaden Muffett and Maria Pia Miglietta Department of Marine Biology, Texas A&M University - Galveston, Galveston, Texas, United States ABSTRACT Jellyfish are known to carry various epibionts, including many of the subphylum Crustacea. However, the associations between gelatinous zooplankton and other invertebrates have been chronically overlooked. Crustacea, a massive clade of economically, ecologically, and culturally important species, includes many taxa that utilize gelatinous zooplankton for food, transport, and protection as both adults and juveniles. Here we compile 211 instances of epifaunal crustaceans recorded on Hydromedusae and Scyphomedusae from a century of literature. These include 78 identified crustacean species in 65 genera across nine orders found upon 37 Hydromedusa species and 48 Scyphomedusae. The crustacean life stage, location, nature of the association with the medusa, years, months, and depths are compiled to form a comprehensive view of the current state of the literature. Additionally, this review highlights areas where the current literature is lacking, particularly noting our poor understanding of the relationships between juvenile crabs of commercially valuable species and medusae. Subjects Ecology, Marine Biology, Zoology Keywords Hydrozoa, Scyphozoa, Crustacea, Association, Commensal, Epifauna, Marine, Jellyfish, Medusa Submitted 17 December 2020 Accepted 24 March 2021 BACKGROUND Published 23 April 2021 An increased focus on ocean climate research in the past 20 years has made clear the Corresponding author fragility of the world’s oceans and the organisms that live within them. The rate at which Kaden Muffett, species are disappearing, undergoing climate-related range fluctuations, and experiencing [email protected] developmental and behavioral changes is unlike anything seen in the time of record Academic editor Antonina Dos Santos (Walther et al., 2002; Guinotte & Fabry, 2008; Comeaux, Allison & Bianchi, 2012). -

`Degraded' RNA Profiles in Arthropoda and Beyond
‘Degraded’ RNA profiles in Arthropoda and beyond Sean D. McCarthy, Michel M. Dugon and Anne Marie Power School of Natural Sciences, Ryan Institute for Environmental, Marine and Energy Research, National University of Ireland Galway, Ireland ABSTRACT The requirement for high quality/non-degraded RNA is essential for an array of molecular biology analyses. When analysing the integrity of rRNA from the barnacle Lepas anatifera (Phylum Arthropoda, Subphylum Crustacea), atypical or sub- optimal rRNA profiles that were apparently degraded were observed on a bioanalyser electropherogram. It was subsequently discovered that the rRNA was not degraded, but arose due to a ‘gap deletion’ (also referred to as ‘hidden break’) in the 28S rRNA. An apparent excision at this site caused the 28S rRNA to fragment under heat- denaturing conditions and migrate along with the 18S rRNA, superficially presenting a ‘degraded’ appearance. Examination of the literature showed similar observations in a small number of older studies in insects; however, reading across multiple disciplines suggests that this is a wider issue that occurs across the Animalia and beyond. The current study shows that the 28S rRNA anomaly goes far beyond insects within the Arthropoda and is widespread within this phylum. We confirm that the anomaly is associated with thermal conversion because gap-deletion patterns were observed in heat-denatured samples but not in gels with formaldehyde-denaturing. Subjects Aquaculture, Fisheries and Fish Science, Genomics, Marine Biology, Taxonomy, Zoology Keywords Hidden break, Denaturing, Taxonomy, Gap deletion, Degraded rNA, Bioanalyser Submitted 4 July 2015 Accepted 4 November 2015 INTRODUCTION Published 1 December 2015 Anomalies in the gel migration of the 28S subunit rRNA in denatured samples have Corresponding author mostly appeared in the older literature (Applebaum, Ebstein& Wyatt, 1966 ; Ishikawa& Anne Marie Power, [email protected] Newburgh, 1972; Fujiwara& Ishikawa, 1986 ). -

Ordens Lepadiformes, Scalpelliformes, Verruciformes E Balaniformes
Revista IDE@ - SEA, nº 99B (30-06-2015): 1–12. ISSN 2386-7183 1 Ibero Diversidad Entomológica @ccesible www.sea-entomologia.org/IDE@ Órdenes Lepadiformes, Scalpelliformes, Clase: Thecostraca Manual Verruciformes y Balaniformes Versión en español CLASSE THECOSTRACA: SUBCLASSE CIRRIPEDIA: SUPERORDEM THORACICA: Ordens Lepadiformes, Scalpelliformes, Verruciformes e Balaniformes Teresa Cruz1,2, Joana N. Fernandes1, Robert J. Van Syoc3 & William A. Newman4 1 MARE – Marine and Environmental Sciences Center, Laboratório de Ciências do Mar, Universidade de Évora, Apartado 190, 7521-903 Sines, Portugal. 2 Departamento de Biologia, Escola de Ciências e Tecnologia, Universidade de Évora, Évora, Portugal. 3 California Academy of Sciences, 55 Music Concourse Drive, San Francisco, CA 94118, USA. 4 Scripps Institution of Oceanography, University of California San Diego, La Jolla CA 92093, USA. [email protected] 1. Breve definição do grupo e principais características diagnosticantes A superordem Thoracica pertence à classe Thecostraca e à subclasse Cirripedia (cirri / cirros - apêndices torácicos modificados). Os cirrípedes (“barnacles” em inglês) são crustáceos cujos adultos são geralmente sésseis e vivem fixos a um substrato duro ou a outros organismos. O corpo dos cirrípedes é envolvido por uma carapaça (manto) que na maioria das formas segrega uma concha calcária, o que levou, no século XIX, à sua identificação incorreta como moluscos. Os cirrípedes também incluem as superordens Acrothoracica (“burrowing barnacles”, vivem em buracos de substrato calcário e têm os apêndices torácicos localizados na extremidade do tórax) e Rhizocephala (parasitas muito modificados, sem apêndices torácicos), podendo os Thoracica (apêndices torácicos presentes ao longo de um tórax bem desenvolvido) ser considerados como a superordem mais importante dos Cirripedia por serem o grupo mais diversificado, abundante e conspícuo, já tendo sido designados como os verdadeiros cirrípedes (“true barnacles”) (Newman & Abbott, 1980). -

Invertebrate Collection Donated by Professor Dr. Ion Cantacuzino To
Travaux du Muséum National d’Histoire Naturelle «Grigore Antipa» Vol. 59 (1) pp. 7–30 DOI: 10.1515/travmu-2016-0013 Research paper Invertebrate Collection Donated by Professor Dr. Ion Cantacuzino to “Grigore Antipa” National Museum of Natural History from Bucharest Iorgu PETRESCU*, Ana–Maria PETRESCU ”Grigore Antipa” National Museum of Natural History, 1 Kiseleff Blvd., 011341 Bucharest 1, Romania. *corresponding author, e–mail: [email protected] Received: November 16, 2015; Accepted: April 18, 2016; Available online: June 28, 2016; Printed: June 30, 2016 Abstract. The catalogue of the invertebrate collection donated by Prof. Dr. Ion Cantacuzino represents the first detailed description of this historical act. The early years of Prof. Dr. Ion Cantacuzino’s career are dedicated to natural sciences, collecting and drawing of marine invertebrates followed by experimental studies. The present paper represents gathered data from Grigore Antipa 1931 inventory, also from the original handwritten labels. The specimens were classified by current nomenclature. The present donation comprises 70 species of Protozoa, Porifera, Coelenterata, Mollusca, Annelida, Bryozoa, Sipuncula, Arthropoda, Chaetognatha, Echinodermata, Tunicata and Chordata.. The specimens were collected from the North West of the Mediterranean Sea (Villefranche–sur–Mer) and in 1899 were donated to the Museum of Natural History from Bucharest. The original catalogue of the donation was lost and along other 27 specimens. This contribution represents an homage to Professor’s Dr. Cantacuzino generosity and withal restoring this donation to its proper position on cultural heritage hallway. Key words. Ion Cantacuzino, donation, collection, marine invertebrates, Mediterranean Sea, Villefranche–sur–Mer, France. INTRODUCTION The name of Professor Dr.