Arthropoda, Cirripedia: the Barnacles Andrew J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Cirripedia : Thoracica) Over the Body of a Sea Snake, Laticauda Title Semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan
Distribution of Two Species of Conchoderma (Cirripedia : Thoracica) over the Body of a Sea Snake, Laticauda Title semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan Yamato, Shigeyuki; Yusa, Yoichi; Tanase, Hidetomo; Tanase, Author(s) Hidetomo PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1996), 37(3-6): 337-343 Issue Date 1996-12-25 URL http://hdl.handle.net/2433/176259 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Pub!. Seto Mar. Bioi. Lab., 37(3/6): 337-343, 1996 337 Distribution of Two Species of Conchoderma (Cirripedia: Thoracica) over the Body of a Sea Snake, Laticauda semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan SHIGEYUKI YAMATO, YOICHI YUSA and HIDETOMO TANASE Seto Marine Biological Laboratory, Kyoto University, Shirahama, Wakayama 649-22, Japan Abstract Two species of Conchoderma were found on a sea snake, Laticauda semifas ciata (Reinwardt), collected on the west coast of the Kii Peninsula. A total of 223 individuals of C. virgatum and 6 of C. hunteri in 19 clumps were attached to the snake's body. The barnacles ranged in size from 1.4 mm (cypris larvae) to 18.2 mm in capitulum length in C. virgatum, and from 10.7 to 14.4 mm in C. hunteri. The size of the smallest gravid individuals in both species was between 10 and 11 mm. The distribution of C. virgatum on the snake was non-random both longitudinally and dorso-ventrally, with more barnacles in the posterior region and on the ventral side of the snake, respectively. The proportion of gravid individuals increased towards the tail. -

Appendix 1 Table A1
OIK-00806 Kordas, R. L., Dudgeon, S., Storey, S., and Harley, C. D. G. 2014. Intertidal community responses to field-based experimental warming. – Oikos doi: 10.1111/oik.00806 Appendix 1 Table A1. Thermal information for invertebrate species observed on Salt Spring Island, BC. Species name refers to the species identified in Salt Spring plots. If thermal information was unavailable for that species, information for a congeneric from same region is provided (species in parentheses). Response types were defined as; optimum - the temperature where a functional trait is maximized; critical - the mean temperature at which individuals lose some essential function (e.g. growth); lethal - temperature where a predefined percentage of individuals die after a fixed duration of exposure (e.g., LT50). Population refers to the location where individuals were collected for temperature experiments in the referenced study. Distribution and zonation information retrieved from (Invertebrates of the Salish Sea, EOL) or reference listed in entry below. Other abbreviations are: n/g - not given in paper, n/d - no data for this species (or congeneric from the same geographic region). Invertebrate species Response Type Temp. Medium Exposure Population Zone NE Pacific Distribution Reference (°C) time Amphipods n/d for NE low- many spp. worldwide (Gammaridea) Pacific spp high Balanus glandula max HSP critical 33 air 8.5 hrs Charleston, OR high N. Baja – Aleutian Is, Berger and Emlet 2007 production AK survival lethal 44 air 3 hrs Vancouver, BC Liao & Harley unpub Chthamalus dalli cirri beating optimum 28 water 1hr/ 5°C Puget Sound, WA high S. CA – S. Alaska Southward and Southward 1967 cirri beating lethal 35 water 1hr/ 5°C survival lethal 46 air 3 hrs Vancouver, BC Liao & Harley unpub Emplectonema gracile n/d low- Chile – Aleutian Islands, mid AK Littorina plena n/d high Baja – S. -

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -
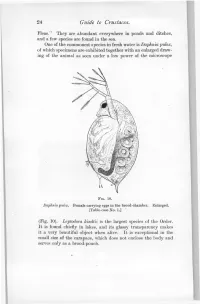
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

Does the Acorn Barnacle Balanus Glandula Exhibit Predictable Gradients in Metabolic Performance Across the Intertidal Zone?
Warren J. Baker Endowment for Excellence in Project-Based Learning Robert D. Koob Endowment for Student Success FINAL REPORT Final reports will be published on the Cal Poly Digital Commons website(http://digitalcommons.calpoly.edu). I. Project Title Does the acorn barnacle Balanus glandula exhibit predictable gradients in metabolic performance across the intertidal zone? II. Project Completion Date 2/2019 III. Student(s), Department(s), and Major(s) (1) Kali Horn, Biological Sciences Department, M.S. Biological Sciences IV. Faculty Advisor and Department Kristin Hardy, Biological Sciences Department V. Cooperating Industry, Agency, Non-Profit, or University Organization(s) NA VI. Executive Summary Using funding from the Baker-Koob endowment, I successfully completed an experiment looking at metabolic variation in the common acorn barnacle, Balanus glandula, across tidal heights. We characterized the temperature profile across the zone as well to have data explicitly describing the abiotic variability from the upper intertidal zone to the low. Myself and many undergraduates collected barnacles from the top middle and bottom of the B.glandula distribution and ran the individuals through a suite of experiments to characterize the ‘metabolic phenotype’ . We defined the metabolic phenotype with a comprehensive suite of biochemical (e.g., citrate synthase and lactate dehydrogenase activity), physiological (VO2, aerobic scope) and behavioral (feeding rate) indices of metabolism. After removal from the intertidal zone, barnacles were placed in our intermittent respirometry system to calculate an average oxygen consumption rate over a 24h period. During the first two hours of the experiment, I conducted a behavioral observation to determine overall activity and feeding rate. -

I © Copyright 2015 Kevin R. Turner
© Copyright 2015 Kevin R. Turner i Effects of fish predation on benthic communities in the San Juan Archipelago Kevin R. Turner A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: Kenneth P. Sebens, Chair Megan N. Dethier Daniel E. Schindler Program Authorized to Offer Degree: Biology ii University of Washington Abstract Effects of fish predation on benthic communities in the San Juan Archipelago Kevin R. Turner Chair of the Supervisory Committee: Professor Kenneth P. Sebens Department of Biology Predation is a strong driver of community assembly, particularly in marine systems. Rockfish and other large fishes are the dominant predators in the rocky subtidal habitats of the San Juan Archipelago in NW Washington State. Here I examine the consumptive effects of these predatory fishes, beginning with a study of rockfish diet, and following with tests of the direct influence of predation on prey species and the indirect influence on other community members. In the first chapter I conducted a study of the diet of copper rockfish. Food web models benefit from recent and local data, and in this study I compared my findings with historic diet data from the Salish Sea and other localities along the US West Coast. Additionally, non-lethal methods of diet sampling are necessary to protect depleted rockfish populations, and I successfully used gastric lavage to sample these fish. Copper rockfish from this study fed primarily on shrimp and other demersal crustaceans, and teleosts made up a very small portion of their diet. Compared to previous studies, I found much higher consumption of shrimp and much iii lower consumption of teleosts, a difference that is likely due in part to geographic or temporal differences in prey availability. -

1 Crustaceans in Cold Seep Ecosystems: Fossil Record, Geographic Distribution, Taxonomic Composition, 2 and Biology 3 4 Adiël A
1 Crustaceans in cold seep ecosystems: fossil record, geographic distribution, taxonomic composition, 2 and biology 3 4 Adiël A. Klompmaker1, Torrey Nyborg2, Jamie Brezina3 & Yusuke Ando4 5 6 1Department of Integrative Biology & Museum of Paleontology, University of California, Berkeley, 1005 7 Valley Life Sciences Building #3140, Berkeley, CA 94720, USA. Email: [email protected] 8 9 2Department of Earth and Biological Sciences, Loma Linda University, Loma Linda, CA 92354, USA. 10 Email: [email protected] 11 12 3South Dakota School of Mines and Technology, Rapid City, SD 57701, USA. Email: 13 [email protected] 14 15 4Mizunami Fossil Museum, 1-47, Yamanouchi, Akeyo-cho, Mizunami, Gifu, 509-6132, Japan. 16 Email: [email protected] 17 18 This preprint has been submitted for publication in the Topics in Geobiology volume “Ancient Methane 19 Seeps and Cognate Communities”. Specimen figures are excluded in this preprint because permissions 20 were only received for the peer-reviewed publication. 21 22 Introduction 23 24 Crustaceans are abundant inhabitants of today’s cold seep environments (Chevaldonné and Olu 1996; 25 Martin and Haney 2005; Karanovic and Brandão 2015), and could play an important role in structuring 26 seep ecosystems. Cold seeps fluids provide an additional source of energy for various sulfide- and 27 hydrocarbon-harvesting bacteria, often in symbiosis with invertebrates, attracting a variety of other 28 organisms including crustaceans (e.g., Levin 2005; Vanreusel et al. 2009; Vrijenhoek 2013). The 29 percentage of crustaceans of all macrofaunal specimens is highly variable locally in modern seeps, from 30 0–>50% (Dando et al. 1991; Levin et al. -

Balanus Glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia
Phylum: Arthropoda, Crustacea Balanus glandula Class: Multicrustacea, Hexanauplia, Thecostraca, Cirripedia Order: Thoracica, Sessilia, Balanomorpha Acorn barnacle Family: Balanoidea, Balanidae, Balaninae Description (the plate overlapping plate edges) and radii Size: Up to 3 cm in diameter, but usually (the plate edge marked off from the parietes less than 1.5 cm (Ricketts and Calvin 1971; by a definite change in direction of growth Kozloff 1993). lines) (Fig. 3b) (Newman 2007). The plates Color: Shell usually white, often irregular themselves include the carina, the carinola- and color varies with state of erosion. Cirri teral plates and the compound rostrum (Fig. are black and white (see Plate 11, Kozloff 3). 1993). Opercular Valves: Valves consist of General Morphology: Members of the Cirri- two pairs of movable plates inside the wall, pedia, or barnacles, can be recognized by which close the aperture: the tergum and the their feathery thoracic limbs (called cirri) that scutum (Figs. 3a, 4, 5). are used for feeding. There are six pairs of Scuta: The scuta have pits on cirri in B. glandula (Fig. 1). Sessile barna- either side of a short adductor ridge (Fig. 5), cles are surrounded by a shell that is com- fine growth ridges, and a prominent articular posed of a flat basis attached to the sub- ridge. stratum, a wall formed by several articulated Terga: The terga are the upper, plates (six in Balanus species, Fig. 3) and smaller plate pair and each tergum has a movable opercular valves including terga short spur at its base (Fig. 4), deep crests for and scuta (Newman 2007) (Figs. -

Marine Information Network Information on the Species and Habitats Around the Coasts and Sea of the British Isles
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Montagu's stellate barnacle (Chthamalus montagui) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Karen Riley 2002-01-28 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1322]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Riley, K. 2002. Chthamalus montagui Montagu's stellate barnacle. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1322.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2002-01-28 Montagu's stellate barnacle (Chthamalus montagui) - Marine Life Information Network See online review for distribution map Close up of Chthamalus montagui from High Water of Spring Tide level seen dry. -

Balanus Trigonus
Nauplius ORIGINAL ARTICLE THE JOURNAL OF THE Settlement of the barnacle Balanus trigonus BRAZILIAN CRUSTACEAN SOCIETY Darwin, 1854, on Panulirus gracilis Streets, 1871, in western Mexico e-ISSN 2358-2936 www.scielo.br/nau 1 orcid.org/0000-0001-9187-6080 www.crustacea.org.br Michel E. Hendrickx Evlin Ramírez-Félix2 orcid.org/0000-0002-5136-5283 1 Unidad académica Mazatlán, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. A.P. 811, Mazatlán, Sinaloa, 82000, Mexico 2 Oficina de INAPESCA Mazatlán, Instituto Nacional de Pesca y Acuacultura. Sábalo- Cerritos s/n., Col. Estero El Yugo, Mazatlán, 82112, Sinaloa, Mexico. ZOOBANK http://zoobank.org/urn:lsid:zoobank.org:pub:74B93F4F-0E5E-4D69- A7F5-5F423DA3762E ABSTRACT A large number of specimens (2765) of the acorn barnacle Balanus trigonus Darwin, 1854, were observed on the spiny lobster Panulirus gracilis Streets, 1871, in western Mexico, including recently settled cypris (1019 individuals or 37%) and encrusted specimens (1746) of different sizes: <1.99 mm, 88%; 1.99 to 2.82 mm, 8%; >2.82 mm, 4%). Cypris settled predominantly on the carapace (67%), mostly on the gastric area (40%), on the left or right orbital areas (35%), on the head appendages, and on the pereiopods 1–3. Encrusting individuals were mostly small (84%); medium-sized specimens accounted for 11% and large for 5%. On the cephalothorax, most were observed in branchial (661) and orbital areas (240). Only 40–41 individuals were found on gastric and cardiac areas. Some individuals (246), mostly small (95%), were observed on the dorsal portion of somites. -

Appendix 3 Marine Spcies Lists
Appendix 3 Marine Species Lists with Abundance and Habitat Notes for Provincial Helliwell Park Marine Species at “Wall” at Flora Islet and Reef Marine Species at Norris Rocks Marine Species at Toby Islet Reef Marine Species at Maude Reef, Lambert Channel Habitats and Notes of Marine Species of Helliwell Provincial Park Helliwell Provincial Park Ecosystem Based Plan – March 2001 Marine Species at wall at Flora Islet and Reef Common Name Latin Name Abundance Notes Sponges Cloud sponge Aphrocallistes vastus Abundant, only local site occurance Numerous, only local site where Chimney sponge, Boot sponge Rhabdocalyptus dawsoni numerous Numerous, only local site where Chimney sponge, Boot sponge Staurocalyptus dowlingi numerous Scallop sponges Myxilla, Mycale Orange ball sponge Tethya californiana Fairly numerous Aggregated vase sponge Polymastia pacifica One sighting Hydroids Sea Fir Abietinaria sp. Corals Orange sea pen Ptilosarcus gurneyi Numerous Orange cup coral Balanophyllia elegans Abundant Zoanthids Epizoanthus scotinus Numerous Anemones Short plumose anemone Metridium senile Fairly numerous Giant plumose anemone Metridium gigantium Fairly numerous Aggregate green anemone Anthopleura elegantissima Abundant Tube-dwelling anemone Pachycerianthus fimbriatus Abundant Fairly numerous, only local site other Crimson anemone Cribrinopsis fernaldi than Toby Islet Swimming anemone Stomphia sp. Fairly numerous Jellyfish Water jellyfish Aequoria victoria Moon jellyfish Aurelia aurita Lion's mane jellyfish Cyanea capillata Particuilarly abundant -

Using Growth Rates to Estimate Age of the Sea Turtle Barnacle Chelonibia Testudinaria
Mar Biol (2017) 164:222 DOI 10.1007/s00227-017-3251-5 SHORT NOTE Using growth rates to estimate age of the sea turtle barnacle Chelonibia testudinaria Sophie A. Doell1 · Rod M. Connolly1 · Colin J. Limpus2 · Ryan M. Pearson1 · Jason P. van de Merwe1 Received: 4 May 2017 / Accepted: 20 October 2017 © Springer-Verlag GmbH Germany 2017 Abstract Epibionts can serve as valuable ecological indi- may live for up to 2 years, means that these barnacles may cators, providing information about the behaviour or health serve as interesting ecological indicators over this period. of the host. The use of epibionts as indicators is, however, In turn, this information may be used to better understand often limited by a lack of knowledge about the basic ecology the movement and habitat use of their sea turtle hosts, ulti- of these ‘hitchhikers’. This study investigated the growth mately improving conservation and management of these rates of a turtle barnacle, Chelonibia testudinaria, under threatened animals. natural conditions, and then used the resulting growth curve to estimate the barnacle’s age. Repeat morphomet- ric measurements (length and basal area) on 78 barnacles Introduction were taken, as host loggerhead turtles (Caretta caretta) laid successive clutches at Mon Repos, Australia, during the Sea turtles are known to host diverse communities of plants 2015/16 nesting season. Barnacles when frst encountered and invertebrate animals (Caine 1986; Kitsos et al. 2005; ranged in size from 3.7 to 62.9 mm, and were recaptured Robinson et al. 2017). Past analyses of the size, abundance, between 12 and 56 days later.