A Checklist of Turtle and Whale Barnacles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

15 Sea Turtle Epibiosis
15 Sea Turtle Epibiosis Michael G. Frick and joseph B. Pfaller CONTENTS 15. I Introduction .......................................................................................................................... 399 15.2 Common Forms .................................................................................................................... 401 15.2.1 Sessile Forms ............................................................................................................ 401 15.2.2 Sedentary Forms ....................................................................................................... 401 15.2.3 Motile Forms ............................................................................................................ 401 15.3 Communities and Community Dynamics ............................................................................ 402 15.3.1 Pelagic/Oceanic Communities .................................................................................. 402 15.3.2 Benthic/Neritic Communities ................................................................................... 402 15.3.3 Obligate Communities .............................................................................................. 403 15.3.4 Community Distribution ........................................................................................... 403 15.3.5 Community Succession ............................................................................................ 404 15.4 Ecological Interactions ........................................................................................................ -

(Cirripedia : Thoracica) Over the Body of a Sea Snake, Laticauda Title Semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan
Distribution of Two Species of Conchoderma (Cirripedia : Thoracica) over the Body of a Sea Snake, Laticauda Title semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan Yamato, Shigeyuki; Yusa, Yoichi; Tanase, Hidetomo; Tanase, Author(s) Hidetomo PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1996), 37(3-6): 337-343 Issue Date 1996-12-25 URL http://hdl.handle.net/2433/176259 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Pub!. Seto Mar. Bioi. Lab., 37(3/6): 337-343, 1996 337 Distribution of Two Species of Conchoderma (Cirripedia: Thoracica) over the Body of a Sea Snake, Laticauda semifasciata (Reinwardt), from the Kii Peninsula, Southwestern Japan SHIGEYUKI YAMATO, YOICHI YUSA and HIDETOMO TANASE Seto Marine Biological Laboratory, Kyoto University, Shirahama, Wakayama 649-22, Japan Abstract Two species of Conchoderma were found on a sea snake, Laticauda semifas ciata (Reinwardt), collected on the west coast of the Kii Peninsula. A total of 223 individuals of C. virgatum and 6 of C. hunteri in 19 clumps were attached to the snake's body. The barnacles ranged in size from 1.4 mm (cypris larvae) to 18.2 mm in capitulum length in C. virgatum, and from 10.7 to 14.4 mm in C. hunteri. The size of the smallest gravid individuals in both species was between 10 and 11 mm. The distribution of C. virgatum on the snake was non-random both longitudinally and dorso-ventrally, with more barnacles in the posterior region and on the ventral side of the snake, respectively. The proportion of gravid individuals increased towards the tail. -
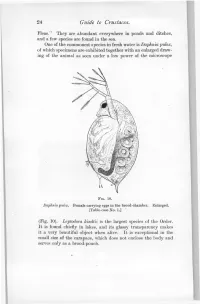
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

The 17Th International Colloquium on Amphipoda
Biodiversity Journal, 2017, 8 (2): 391–394 MONOGRAPH The 17th International Colloquium on Amphipoda Sabrina Lo Brutto1,2,*, Eugenia Schimmenti1 & Davide Iaciofano1 1Dept. STEBICEF, Section of Animal Biology, via Archirafi 18, Palermo, University of Palermo, Italy 2Museum of Zoology “Doderlein”, SIMUA, via Archirafi 16, University of Palermo, Italy *Corresponding author, email: [email protected] th th ABSTRACT The 17 International Colloquium on Amphipoda (17 ICA) has been organized by the University of Palermo (Sicily, Italy), and took place in Trapani, 4-7 September 2017. All the contributions have been published in the present monograph and include a wide range of topics. KEY WORDS International Colloquium on Amphipoda; ICA; Amphipoda. Received 30.04.2017; accepted 31.05.2017; printed 30.06.2017 Proceedings of the 17th International Colloquium on Amphipoda (17th ICA), September 4th-7th 2017, Trapani (Italy) The first International Colloquium on Amphi- Poland, Turkey, Norway, Brazil and Canada within poda was held in Verona in 1969, as a simple meet- the Scientific Committee: ing of specialists interested in the Systematics of Sabrina Lo Brutto (Coordinator) - University of Gammarus and Niphargus. Palermo, Italy Now, after 48 years, the Colloquium reached the Elvira De Matthaeis - University La Sapienza, 17th edition, held at the “Polo Territoriale della Italy Provincia di Trapani”, a site of the University of Felicita Scapini - University of Firenze, Italy Palermo, in Italy; and for the second time in Sicily Alberto Ugolini - University of Firenze, Italy (Lo Brutto et al., 2013). Maria Beatrice Scipione - Stazione Zoologica The Organizing and Scientific Committees were Anton Dohrn, Italy composed by people from different countries. -

Epibiotic Associates of Oceanic-Stage Loggerhead Turtles from the Southeastern North Atlantic
Acknowledgements We thank the biology students of the occasional leatherback nests in Brazil. Marine Turtle Federal University of Paraíba (Pablo Riul, Robson G. dos Newsletter 96:13-16. Santos, André S. dos Santos, Ana C. G. P. Falcão, Stenphenson Abrantes, MS Elaine Elloy), the marathon MARCOVALDI, M. Â. & G. G. MARCOVALDI. 1999. Marine runner José A. Nóbrega, and the journalist Germana turtles of Brazil: the history and structure of Projeto Bronzeado for the volunteer field work; the Fauna department TAMAR-IBAMA. Biological Conservation 91:35-41. of IBAMA/PB and Jeremy and Diana Jeffers for kindly MARCOVALDI, M.Â., C.F. VIEITAS & M.H. GODFREY. 1999. providing photos, and also Alice Grossman for providing Nesting and conservation management of hawksbill turtles the TAMAR protocols. The manuscript benefited from the (Eretmochelys imbricata) in northern Bahia, Brazil. comments of two referees. Chelonian Conservation and Biology 3:301-307. BARATA, P.C.R. & F.F.C. FABIANO. 2002. Evidence for SAMPAIO, C.L.S. 1999. Dermochelys coriacea (Leatherback leatherback sea turtle (Dermochelys coriacea) nesting in sea turtle), accidental capture. Herpetological Review Arraial do Cabo, state of Rio de Janeiro, and a review of 30:38-39. Epibiotic Associates of Oceanic-Stage Loggerhead Turtles from the Southeastern North Atlantic Michael G. Frick1, Arnold Ross2, Kristina L. Williams1, Alan B. Bolten3, Karen A. Bjorndal3 & Helen R. Martins4 1 Caretta Research Project, P.O. Box 9841, Savannah, Georgia 31412 USA. (E-mail: [email protected]) 2 Scripps Institution of Oceanography, Marine Biology Research Division, La Jolla, California 92093-0202, USA, (E-mail: [email protected]) 3 Archie Carr Center for Sea Turtle Research and Department of Zoology, University of Florida, P.O. -

Gray Whales in the North Pacific: History, Biology, and Current Research
Gray Whales in the North Pacific: History, biology, and current research Aimée Lang Marine Mammal and Turtle Division Southwest Fisheries Science Center 13 October 2015 Photo courtesy of San Diego Natural History Museum Whalers W. Perryman Photo credit: Wayne Perryman, SWFSC Overview: • Taxonomic history • Physical description • Gray whale biology and life history • How we study gray whales and what we’ve learned! Taxonomic history: • First described based on subfossil remains from the coast of Sweden (Lilljeborg 1861) • “Eschrichtius” – named after a Danish zoologist (Dr. Daniel Eschricht) who was the first to suggest the remains might be from a new genus and family • “robustus” – Latin for strong Relationship to other baleen whales: • Fossil record is generally sparse but suggests higher diversity in the past • Today the gray whale is the only living species in its genus and family • Although traditionally considered morphologically distinct from the rorqual whales, molecular analyses indicate that gray whales are closely related to balaenopterid whales Sasaki et al. 2005, mitogenome analysis Physical characteristics: • Heart-shaped blow • Mottled gray and white coloration • Dorsal hump followed by series of 6 to 12 “knuckles” • Yellowish white baleen • Fewest baleen plates of any mysticete (130-180 plates on each side of mouth) • 2-5 throat grooves Size: • Adult body 11 to 15 m and weigh 45,000 kg • Females are larger than males • Calves 4.6 to 4.9 m at birth and weigh 700-900 kg Gray whale barnacles (Cryptolepas rhachianecti): • Considered -

Issue Number 118 October 2007 ISSN 0839-7708 in THIS
Issue Number 118 October 2007 Green turtle hatchling from Turkey with extra carapacial scutes (see pp. 6-8). Photo by O. Türkozan IN THIS ISSUE: Editorial: Conservation Conflicts, Conflicts of Interest, and Conflict Resolution: What Hopes for Marine Turtle Conservation?..........................................................................................L.M. Campbell Articles: From Hendrickson (1958) to Monroe & Limpus (1979) and Beyond: An Evaluation of the Turtle Barnacle Tubicinella cheloniae.........................................................A. Ross & M.G. Frick Nest relocation as a conservation strategy: looking from a different perspective...................O. Türkozan & C. Yılmaz Linking Micronesia and Southeast Asia: Palau Sea Turtle Satellite Tracking and Flipper Tag Returns......S. Klain et al. Morphometrics of the Green Turtle at the Atol das Rocas Marine Biological Reserve, Brazil...........A. Grossman et al. Notes: Epibionts of Olive Ridley Turtles Nesting at Playa Ceuta, Sinaloa, México...............................L. Angulo-Lozano et al. Self-Grooming by Loggerhead Turtles in Georgia, USA..........................................................M.G. Frick & G. McFall IUCN-MTSG Quarterly Report Announcements News & Legal Briefs Recent Publications Marine Turtle Newsletter No. 118, 2007 - Page 1 ISSN 0839-7708 Editors: Managing Editor: Lisa M. Campbell Matthew H. Godfrey Michael S. Coyne Nicholas School of the Environment NC Sea Turtle Project A321 LSRC, Box 90328 and Earth Sciences, Duke University NC Wildlife Resources Commission Nicholas School of the Environment 135 Duke Marine Lab Road 1507 Ann St. and Earth Sciences, Duke University Beaufort, NC 28516 USA Beaufort, NC 28516 USA Durham, NC 27708-0328 USA E-mail: [email protected] E-mail: [email protected] E-mail: [email protected] Fax: +1 252-504-7648 Fax: +1 919 684-8741 Founding Editor: Nicholas Mrosovsky University of Toronto, Canada Editorial Board: Brendan J. -

Using Growth Rates to Estimate Age of the Sea Turtle Barnacle Chelonibia Testudinaria
Mar Biol (2017) 164:222 DOI 10.1007/s00227-017-3251-5 SHORT NOTE Using growth rates to estimate age of the sea turtle barnacle Chelonibia testudinaria Sophie A. Doell1 · Rod M. Connolly1 · Colin J. Limpus2 · Ryan M. Pearson1 · Jason P. van de Merwe1 Received: 4 May 2017 / Accepted: 20 October 2017 © Springer-Verlag GmbH Germany 2017 Abstract Epibionts can serve as valuable ecological indi- may live for up to 2 years, means that these barnacles may cators, providing information about the behaviour or health serve as interesting ecological indicators over this period. of the host. The use of epibionts as indicators is, however, In turn, this information may be used to better understand often limited by a lack of knowledge about the basic ecology the movement and habitat use of their sea turtle hosts, ulti- of these ‘hitchhikers’. This study investigated the growth mately improving conservation and management of these rates of a turtle barnacle, Chelonibia testudinaria, under threatened animals. natural conditions, and then used the resulting growth curve to estimate the barnacle’s age. Repeat morphomet- ric measurements (length and basal area) on 78 barnacles Introduction were taken, as host loggerhead turtles (Caretta caretta) laid successive clutches at Mon Repos, Australia, during the Sea turtles are known to host diverse communities of plants 2015/16 nesting season. Barnacles when frst encountered and invertebrate animals (Caine 1986; Kitsos et al. 2005; ranged in size from 3.7 to 62.9 mm, and were recaptured Robinson et al. 2017). Past analyses of the size, abundance, between 12 and 56 days later. -

Redalyc.Distribution Patterns of the Barnacle, Chelonibia Testudinaria
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Nájera-Hillman, Eduardo; Bass, Julie B.; Buckham, Shannon Distribution patterns of the barnacle, Chelonibia testudinaria, on juvenile green turtles (Chelonia mydas) in Bahia Magdalena, Mexico Revista Mexicana de Biodiversidad, vol. 83, núm. 4, diciembre, 2012, pp. 1171-1179 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42525092012 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 83: 1171-1179, 2012 DOI: 10.7550/rmb.27444 Distribution patterns of the barnacle, Chelonibia testudinaria, on juvenile green turtles (Chelonia mydas) in Bahia Magdalena, Mexico Patrones de distribución del balano, Chelonibia testudinaria, en tortugas verdes (Chelonia mydas) juveniles en bahía Magdalena, México Eduardo Nájera-Hillman1 , Julie B. Bass2,3 and Shannon Buckham2,4 1COSTASALVAjE A.C. Las Dunas #160-203. Fracc. Playa Ensenada. 22880 Ensenada, Baja California, México. 2Center for Coastal Studies-The School for Field Studies. 23740 Puerto San Carlos, Baja California Sur, México. 3Wellesley College, 21 Wellesley College Rd., Wellesley, MA 02481 USA. 4Whitman College, 345 Boyer Ave. Walla Walla, WA 99362 USA. [email protected] Abstract. The barnacle, Chelonibia testudinaria, is an obligate commensal of sea turtles that may show population variability according to the physical characteristics of the environment and properties of turtle hosts; therefore, we characterized the distributional patterns and the potential effects on health of C. -

Diversity of Tanaidacea (Crustacea: Peracarida) in the World's Oceans - How Far Have We Come?
The University of Southern Mississippi The Aquila Digital Community Faculty Publications 4-1-2012 Diversity of Tanaidacea (Crustacea: Peracarida) in the World's Oceans - How Far Have We Come? Gary Anderson University of Southern Mississippi, [email protected] Magdalena Blazewicz-Paszkowycz University of Łódź, [email protected] Roger Bamber Artoo Marine Biology Consultants, [email protected] Follow this and additional works at: https://aquila.usm.edu/fac_pubs Part of the Marine Biology Commons Recommended Citation Anderson, G., Blazewicz-Paszkowycz, M., Bamber, R. (2012). Diversity of Tanaidacea (Crustacea: Peracarida) in the World's Oceans - How Far Have We Come?. PLoS One, 7(4), 1-11. Available at: https://aquila.usm.edu/fac_pubs/160 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Faculty Publications by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. Diversity of Tanaidacea (Crustacea: Peracarida) in the World’s Oceans – How Far Have We Come? Magdalena Blazewicz-Paszkowycz1*, Roger Bamber2, Gary Anderson3 1 Department of Polar Biology and Oceanobiology, University of Ło´dz´,Ło´dz´, Poland, 2 Artoo Marine Biology Consultants, Ocean Quay Marina, Southampton, Hants, United Kingdom, 3 Department of Biological Sciences, University of Southern Mississippi, Hattiesburg, Mississippi, United States of America Abstract Tanaidaceans are small peracarid crustaceans which occur in all marine habitats, over the full range of depths, and rarely into fresh waters. Yet they have no obligate dispersive phase in their life-cycle. Populations are thus inevitably isolated, and allopatric speciation and high regional diversity are inevitable; cosmopolitan distributions are considered to be unlikely or non-existent. -

Surveys of the Sea Snakes and Sea Turtles on Reefs of the Sahul Shelf
Surveys of the Sea Snakes and Sea Turtles on Reefs of the Sahul Shelf Monitoring Program for the Montara Well Release Timor Sea MONITORING STUDY S6 SEA SNAKES / TURTLES Dr Michael L Guinea School of Environment Faculty of Engineering, Health, Science and the Environment Charles Darwin University Darwin 0909 Northern Territory Draft Final Report 2012-2013 Acknowledgements: Two survey by teams of ten and eleven people respectively housed on one boat and operating out of three tenders for most of the daylight hours for 20 days and covering over 2500 km of ocean can only succeed with enthusiastic members, competent and obliging crew and good organisation. I am indebted to my team members whose names appear in the personnel list. I thank Drs Arne Rasmussen and Kate Sanders who gave their time and shared their knowledge and experiences. I thank the staff at Pearl Sea Coastal Cruises for their organisation and forethought. In particular I thank Alice Ralston who kept us on track and informed. The captains Ben and Jeff and Engineer Josh and the coxswains Riley, Cam, Blade and Brad; the Chef Stephen and hostesses Sunny and Ellen made the trips productive, safe and enjoyable. I thank the Department of Environment and Conservation WA for scientific permits to enter the reserves of Sandy Islet, Scott Reef and Browse Island. I am grateful to the staff at DSEWPaC, for facilitating and providing the permits to survey sea snakes and marine turtles at Ashmore Reef and Cartier Island. Activities were conducted under Animal Ethics Approval A11028 from Charles Darwin University. Olive Seasnake, Aipysurus laevis, on Seringapatam Reef. -

Remarkable Convergent Evolution in Specialized Parasitic Thecostraca (Crustacea)
Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea) Pérez-Losada, Marcos; Høeg, Jens Thorvald; Crandall, Keith A Published in: BMC Biology DOI: 10.1186/1741-7007-7-15 Publication date: 2009 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Pérez-Losada, M., Høeg, J. T., & Crandall, K. A. (2009). Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea). BMC Biology, 7(15), 1-12. https://doi.org/10.1186/1741-7007-7-15 Download date: 25. Sep. 2021 BMC Biology BioMed Central Research article Open Access Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea) Marcos Pérez-Losada*1, JensTHøeg2 and Keith A Crandall3 Address: 1CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, Portugal, 2Comparative Zoology, Department of Biology, University of Copenhagen, Copenhagen, Denmark and 3Department of Biology and Monte L Bean Life Science Museum, Brigham Young University, Provo, Utah, USA Email: Marcos Pérez-Losada* - [email protected]; Jens T Høeg - [email protected]; Keith A Crandall - [email protected] * Corresponding author Published: 17 April 2009 Received: 10 December 2008 Accepted: 17 April 2009 BMC Biology 2009, 7:15 doi:10.1186/1741-7007-7-15 This article is available from: http://www.biomedcentral.com/1741-7007/7/15 © 2009 Pérez-Losada et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.