Facile Aqueous-Phase Synthesis of Pd-Fept Core-Shell Nanoparticles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

21, 2015, Meiji Gakuin University
The Nineteenth Asian Studies Conference Japan (ASCJ) June 20 – 21, 2015, Meiji Gakuin University SATURDAY JUNE 20 SATURDAY MORNING SESSIONS: 10:00 A.M. – 12:00 P.M. Session 1: Room 1351 Sporting Histories, Mediated Cultures: Women and Sports in Japan Organizer/Chair: Michelle Ho, Stony Brook University 1) Helen Macnaughtan, SOAS, University of London The Oriental Witches: Women, Volleyball and the 1964 Tokyo Olympics 2) Iwona Merklejn, Aoyama Gakuin University Witchcraft or Teamwork? Women’s Volleyball in Japanese Animation and Television Drama 3) Michelle Ho, Stony Brook University Following Nadeshiko Japan on Social Media: Women’s Soccer and Fan Affect 4) Robin Kietlinski, LaGuardia Community College, CUNY Challenging Women: Female Olympians in Twenty-first Century Japan Discussant: Keiko Aiba, Meiji Gakuin University Session 2: Room 1352 New Processes, New Policies? The Politics of Labor Market Reform in Contemporary Japan Organizer/Chair: Steffen Heinrich, German Institute for Japanese Studies (DIJ) 1) Gabriele Vogt, University of Hamburg Health-Caregivers on the Global Labor Market: A Comparative Study of Japan’s Economic Partnership Agreements and Germany’s Triple Win Program 2) Mari Miura, Sophia University Neoliberal Motherhood: Care and Work in the Japanese Welfare State 3) Jiyeoun Song, Seoul National University Precarious Young Workers and Labor Market Reform in Japan 4) Steffen Heinrich, German Institute for Japanese Studies (DIJ) The Politics of Labor Market Reform in Japan and Beyond: Who Decides and Who Cares? Discussant: -

Organizing Committee Important Dates Topics Paper Submission
CALL FOR PAPERS “Smart Technology for an Intelligent Society” Organizing Committee General Co-Chairs Jinwook Burm Sogang University JinGyun Chung Jeonbuk Nat’l University Myung Hoon Sunwoo Ajou University Technical Program Co-Chairs Hanho Lee Inha University KyungKi Kim Daegu University Takao Onoye Osaka University Gabriel Lincon-Mora Georgia Tech. Special Session Co-Chairs Elena Blokhina University College Dublin Ittetsu Taniguchi Osaka University Lan-Da Van Nat’l Chiao Tung University Qiang Li UESTC Ross M. Walker University of Utah Minkyu Je KAIST Tutorial Co-Chairs Massimo Alioto Nat’l University of Singapore Andy Wu Nat’l Taiwan University Samuel Tang (Kea-Tiong Tang) Nat’l Tsing Hua University Hiroo Sekiya Chiba University Jongsun Park Korea University Timothy Constandinou Imperial College of London Important Dates Demo Session Co-Chairs Tobi Delbruck ETH Zurich Ji-Hoon Kim Ewha Womans University Plenary Session Co-Chairs Oct.5 Oct.23 Nov.16 Dec.7 Jan.8 Jan.11 Jan.29 Deog-Kyoon Jeong Seoul Nat’l University 2020 2020 2020 2020 2021 2021 2021 Boris Murmann Stanford University Robert Chen-Hao Chang Nat’l Chung Hsing University Junjin Kong Samsung Electronics Submission 1- Submission Submission Submission Notification of Notification of Final Publicity Co-Chairs deadline for deadline for deadline for deadline for Acceptance Acceptance Submission Hadi Heidari University of Glasgow Special Session Regular Papers CAS Trans. Papers Tutorials (for all papers) (for Demos Deadline Guoxing Wang Shanghai Jiao Tong University Proposals Proposals -

Brown Office of International Programs (OIP) Approved Program List
Brown Office of International Programs (OIP) Approved Program List Country Program Location Program Name Institution Timing Language Argentina Buenos Aires CIEE:IFSA-Butler: Facultad Argentine Latinoamericana Universities de CienciasProgram Sociales & Universidad de Buenos FacultadArgentine Latinoamericana Universities Program de Ciencias Sociales & Universidad Sem/Year Spanish Argentina Buenos Aires Aires de Buenos Aires Sem/Year Spanish Argentina Buenos Aires IES: Advanced Spanish Honors Program Advanced Spanish Honors Program Sem/Year Spanish Argentina Mendoza IFSA-Butler: Universidad Nacional de Cuyo Universidad Nacional de Cuyo Sem/Year Spanish Australia Brisbane Arcadia University: University of Queensland University of Queensland Sem/Year English Australia Brisbane University of Queensland - Direct Enrollment University of Queensland Sem/Year English Australia Brisbane IFSA-Butler: University of Queensland University of Queensland Sem/Year English Australia Cairns SIT: Australia- Rainforest, Reef, and Cultural Ecology SIT Field Station Semester English Australia Canberra Arcadia University: Australian National University Australian National University Sem/Year English Australia Canberra Australian National University - Direct Enrollment Australian National University Sem/Year English Australia Canberra IFSA-Butler: Australian National University Australian National University Sem/Year English Australia Hobart University of Tasmania, Hobart - Direct Enrollment University of Tasmania, Hobart Sem/Year English Australia Hobart IFSA-Butler: -

Summer Programmes 2020 Office of Global Education and Interdisciplinary Studies
Summer Programmes 2020 Office of Global Education and Interdisciplinary Studies GUIDANCE NOTES PROGRAMME LIST Lingnan Programme Host Institution Programme Country Credit(s) details ASIA Akita International University AIU Summer Program 2020 Japan 6 Download Chonnam National University CNU International Summer Session Korea 3 - 6 Download Dankook University International Summer School Korea 3 or 6 Download (Academic Program) Ewha Womans University International Summer College Korea 3 Download (Session 2) Kyung Hee University Global Collaborative 2020 Summer Korea 2 - 6 Download Program Mahidol University International The Belt and Road Study Tour in Thailand Non-credit- Download College Thailand bearing The University of Tokyo GJS Summer Program Japan 2 Download EUROPE Aalto University School of Summer Studies 2020 Finland 6 Download Business, Mikkeli Campus Charles University in Prague, East and Central European Studies Czech Republic 3 or 6 Download Faculty of Arts (Session B) Linköping University LiU Summer Academy 2020 Sweden 3.75 Download Ludwig-Maximilians-Universitat Summer German Language Course Germany 1.5 Download München Tallinn University Tallinn Summer School 2020 Estonia 2.5 or 3.5 Download University of Hradec Králové Czech it Out Summer School 2020 Czech Republic 3 Download Vytautas Magnus University Lithuanian Language and Culture Lithuania 3 Download Summer Course 2020 THE AMERICAS The University of British Vancouver Summer Program (July Canada Non-credit- Download Columbia Program) bearing OR 6 1 January 2020 Summer -

EASP Final Program Book Download
The 13th EASP annual conference: Social Policy and Gender in East Asia Program Book Program Overview Date Time Schedule Venue 08:30-9:00 Registration Posco Bd. B1 Lobby 09:00-10:30 Welcome Ceremony & Plenary Session Posco Bd. B153 10:40-12:10 Session Ⅰ-A, Ⅰ-B, Ⅰ-C, Ⅰ-D Posco Bd. 357-360 Lunch Break 12:10-13:20 Jinseonmi Bd. EASP Executive Committee Meeting Meeting Room Mi-gwan 1 Day 1 13:20-14:50 Session Ⅱ-A, Ⅱ-B, Ⅱ-C, Ⅱ-D, Ⅱ-E Posco Bd. 356-360 15:00-16:30 Session Ⅲ-A, Ⅲ-B, Ⅲ-C, Ⅲ-D, Ⅲ-E Posco Bd. 356-360 16:30-16:50 Tea Break 16:50-18:20 Session Ⅳ-A, Ⅳ-B, Ⅳ-C, Ⅳ-D Posco Bd. 357-360 Welcome Dinner International Education Bd. 18:35-20:00 with Special Korean Classical Music Performance LG Convention Hall 09:00-10:30 Session Ⅴ-A, Ⅴ-B, Ⅴ-C, Ⅴ-D Posco Bd. 357-360 10:30-10:50 Tea Break Day 2 10:50-11:50 Plenary Session Posco Bd. B153 11:50 Closing Ceremony & MOU Ceremony - 1 - 1. Welcome Ceremony & Plenary Session (Day 1) Day 1. 9:00-10:30 Venue Welcome Ceremony Moderator: Sophia Seung-yoon LEE (Chair of Department of Social Welfare, Ewha Womans University) Ÿ Welcoming Address Soondool CHUNG (Leader of BK21 PLUS Project “Social Work Leaders with Creative Capacities in the Changing Society”, Ewha Womans University) Ÿ Welcoming Address Posco Bd. B153 Choong Rai NHO (Director of Institute for Social Welfare Research, Ewha Womans University) Plenary Session Chair: Minah KANG (Ewha Womans University, Republic of Korea) Ÿ The Trilemma of the Care Economy: Possible Paths of Japan Mari MIURA (Sophia University, Japan) Ÿ The East Asian Miracle and the Wollstonecraft Dilemma: In Search for Joohee LEE (Ewha Womans University, a New Paradigm of Gender Egalitarian Social Rights Republic of Korea) 2. -

CV Des 2015 Anitalie
!1Prof. Anita Lie, Ed.D. Widya Mandala Catholic University www.anitalie.com [email protected] Ph: 62-31-599-2432 Cell Ph: 62-81-653-6484 Education Doctor of Education Baylor University, Curriculum and Instruction, 1994 Master of Arts Baylor University, English Literature, 1991 Bachelor of Arts Petra Christian University, English Literature, 1987 Academic Experience Professor Faculty of Teacher Training, University of Widya Mandala, Surabaya, 2008-present Graduate School, University of Widya Mandala, Surabaya, 2008-present Senior Lecturer Faculty of Teacher Training, University of Widya Mandala, Surabaya, 2002-2008 Classroom Supervisor, Education Consultant and Curriculum Designer Several schools and colleges in Indonesia, 1994-present Senior Lecturer Department of English, Petra Christian University, Surabaya, 1987-2002 External Examiner Department of English, Chinese University of Hong Kong, 2002 Visiting Lecturer, SEAMEO Jasper Lecture/Study Tour, 2001 A guest lecture grant by the government of Canada and SEAMEO (South East Asian Ministries of Education Organization) University of British Columbia, Simon Fraser University, McGill University, Chulalongkorn University, University of Brunei Darussalam, University Kebangsaan Malaysia, University Putra Malaysia, Yangon University, Assumption University. Adjunct Senior Lecturer Business Communication and Intercultural Communication International Business Management Program, Petra Christian University, 1999-2002 !1 Teaching Staff Trainer Petra Christian University Teaching Excellence -

Partner Universities in Europe and Middle East
Partner Institutions as of February 2018 Partner Universities in Europe and Middle East Aarhus University Denmark (2) University of Southern Denmark Helsinki Metropolia University Finland (2) University of Vaasa Burgundy School of Business Lumiere University of Lyon 2 Lyon Institute of Political Studies Normandy Business School France (8) Paris Diderot University (Paris 7) Saint-Germain-en-Laye Institute of Political Science University of Lille University of Montpellier European University Viadrina FAU Erlangen Nurnberg Heinrich Heine University Dusseldorf HTW Berlin Germany (8) Johannes Gutenberg University Mainz Ruhr-University Bochum University of Augsburg University of Regensburg Iceland (1) University of Iceland Europe Ireland (1) Dublin City University Ca' Foscari University of Venice Italy (2) University of Parma Latvia (1) University of Latvia Hague University of Applied Sciences Netherland (3) Hanze University of Applied Sciences Radboud University Nijmegen Norwegian University of Science and Technology Norway (2) University of Oslo Cracow University of Economics Poland (2) University of Lodz Spain (1) Pompeu Fabra University Linkoping University Sweden (2) Linnaeus University Cardiff University Keele University SOAS University of London U.K. (7) University of Edinburgh University of Leicester University of Manchester University of Stirling Middle East Turkey (1) Kocaeli University Partner Universities in Asia and Oceania Fudan University Jilin University Shanghai Jiao Tong University China (6) Sichuan University Soochow University -

Faculty-Scholarship-Leaflet-2019.Pdf
United Board Recommended Institutions with English-medium Degree Programs Australia • Macquarie University, Sydney • The University of Melbourne China • Central China Normal University, Wuhan • Fudan University, Shanghai Hong Kong • The Chinese University of Hong Kong • City University of Hong Kong • The Education University of Hong Kong • Hong Kong Baptist University • The Hong Kong Polytechnic University • The Hong Kong University of Science and Technology • Lingnan University • The University of Hong Kong United Board Mission and Identity India • CHRIST (Deemed to be University), Bengaluru The United Board is committed to education that United Board develops the whole person – intellectually, spiritually, Indonesia • Indonesian Consortium for Religious Studies, Yogyakarta and ethically. We draw strength from our Christian identity and values and our collaboration with Asian Faculty Scholarship Japan colleges and universities. Together we prepare • International Christian University, Tokyo individuals for lives of professional and personal • Sophia University, Tokyo fulfilment and meaningful service in community with Program Korea others. • Ewha Womans University, Seoul The United Board is a nongovernmental • Yonsei University, Seoul organization. We are registered as a tax-exempt, Macau nonprofit organization in the United States and as a • The University of Macau public charity in Hong Kong. Philippines For further information about the United Board • Ateneo de Manila University Faculty Scholarship Program, please contact: • De La -
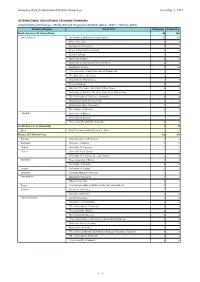
University Data (International Christian University) As of May 1, 2017
University Data (International Christian University) As of May 1, 2017 INTERNATIONAL EDUCATIONAL EXCHANGE PROGRAMS International Exchange / Study Abroad Programs AY2016 (April, 2016 ~ March, 2017) Country/Region University Outgoing Incoming North America (21 Universities) 40 106 United States University of California (9 campuses) 15 65 Duke University 0 0 Georgetown University 0 1 Grand Valley State University 4 2 Guilford College 4 4 Haverford College 0 0 University of Massachusetts at Amherst 2 1 Middlebury College 2 18 The University of North Carolina at Chapel Hill 1 3 The Ohio State University 2 2 University of Pennsylvania 1 0 Pomona College 0 0 Rutgers, The State University of New Jersey 2 3 University at Buffalo, The State University of New York 1 0 The University of Tennessee, Knoxville 0 0 Washington and Lee University 1 1 Washington State University 1 2 The College of Wooster 0 1 Canada University of Alberta 1 2 University of Windsor 1 1 The University of British Columbia 2 0 South America (1 University) 1 0 Chile Pontificia Universidad Catolica de Chile 1 0 Europe (25 Universities) 62 34 Belgium Universié Libre de Bruxelles 1 0 Denmark Univesity of Aarhus 1 0 Finland University of Tampere 3 2 France Université Paris Diderot 1 2 University of Toulouse II - Jean Jaurès 2 3 Germany Free University of Berlin 3 3 University of Münster 0 0 Iceland University of Iceland 2 2 Lithuania Vytautas Magnus University 3 1 Netherlands Maastricht University 3 1 Tilburg University 2 2 Russia The Moscow State Institute of International Relations -

AKIKO WALLEY [email protected]
Walley Curriculum Vitae AKIKO WALLEY [email protected] Employment Maude I. Kerns Associate Professor of Japanese Art 2016-present Maude I. Kerns Assistant Professor of Japanese Art 2009-2016 Department of the History of Art and Architecture College of Design University of Oregon Education PhD Harvard University (Cambridge, MA) 2009 Department: History of Art and Architecture Fields: Japanese and Chinese art history AM Harvard University (Cambridge, MA) 2004 Department: Regional Studies East Asia Fields: Japanese and Chinese culture MA Aoyama Gakuin University (Tokyo, Japan) 2001 Department: Art History Fields: Japanese and Chinese art history BA Aoyama Gakuin University (Tokyo, Japan) 1998 Department: History Fields: Japanese history, Japanese art history Publications (published or forthcoming) Peer-reviewed Monograph Constructing the Dharma King: The Hōryūji Shaka Triad and the Birth of the Prince Shōtoku Cult, Japanese Visual Culture Series, vol. 15 (Leiden: Brill, 2015). Museum Catalogues Evocative Shadows: The Mezzotints of Hamanishi Katsunori, co-edited with Anne Rose Kitagawa (Eugene, OR: Jordan Schnitzer Museum of Art, 2019). Expanding Frontiers: The Jack and Susy Wadsworth Collection of Postwar Japanese Prints, co- edited with Anne Rose Kitagawa (Eugene, OR: Jordan Schnitzer Museum of Art, 2015). Book Chapters/Essays for Exhibition Catalogues “Strike a Chord: The Principle of Resonance in Early East Asian Buddhist Reliquaries,” in Refiguring East Asian Religious Art: Buddhist Devotion and Funerary Practice, edited by Wu Hung and Paul Copp (Chicago: Center for the Art of East Asia, Department of Art History, University of Chicago; Art Media Resources, Inc, 2019), 21-51. 1 Walley Curriculum Vitae “Innovation and Resuscitation: A Short History of Mezzotint,” Evocative Shadows: The Mezzotints of Hamanishi Katsunori, edited by Anne Rose Kitagawa and Akiko Walley (Eugene, OR: Jordan Schnitzer Museum of Art, 2019), 13-19. -

WORLD: the David and Leighan Rinker Center for International Learning 1
WORLD: The David and Leighan Rinker Center for International Learning 1 Exchange programs are designed to engender intercultural WORLD: The David understanding through personal experience and first-hand, high-impact exposure to a country’s language, literature, history, social institutions, and Leighan Rinker and artistic and scientific development. Juniors, seniors, and mature sophomores are encouraged to take part Center for International in one or more study abroad exchange programs. Students may earn one semester or one year of credit at the same tuition cost as study on Learning the home campus, plus international travel, visas, housing, etc. The exchange program at Complutense University of Madrid is supervised Paula Hentz, Director of International Learning by a Resident Director who is a bilingual affiliate of the cooperating Roxanne Lewis, International Student and Scholar Services institution. The programs in all other locations are supervised by the Coordinator international student office of the host university. The overall program Wendy Viggiano, Program Coordinator for International Learning is coordinated by WORLD: The David and Leighan Rinker Center for International Learning. Study Abroad With careful planning, any student is able to study abroad. A study Exchange Programs abroad experience is expected of world languages & cultures and international studies majors. It is required for majors in international Stetson offers students semester and year-long exchange business. Students in the School of Business Administration may opportunities at a range of institutions around the world for in- use approved study abroad programs to fulfill one of their Experiential residence credit. WORLD: The David and Leighan Rinker Center for Learning Requirements. -

Ewha Womans University International Co-Ed Programs Seoul, Korea Contents
OFFICE OF INTERNATIONAL AFFAIRS EWHA WOMANS UNIVERSITY International Co-Ed Programs Seoul, Korea Contents 05 Welcoming Remarks from the President 06 About Ewha Womans University 12 About the Office of International Affairs 14 Semester Abroad at Ewha (Exchange & Visiting Programs) 18 Ewha International Co-Ed Summer College 22 Cultural Excursions 24 Ewha PEACE Buddies 25 On-campus Housing 26 International Partners 32 Fields of Study 36 Research Institutes 38 Campus Map Ewha Campus Complex (ECC) Reading Room Welcoming Remarks from the President Welch-Ryang Auditorium Hello! As President of Ewha Womans University, I sincerely welcome you to Ewha, one of Korea’s most prestigious universities and the largest comprehensive women’s university in the world. Ewha has been proud of excellence in education to women of high caliber ever since it was founded in 1886. Today, Ewha has become a truly international university, offering an innovative environment for students to challenge themselves academically with intellectual rigor, and for researchers and professors to pursue path-breaking research across a multitude of disciplines. As a global university, Ewha has one of Korea’s most comprehensive and well regarded exchange networks, partnering with more than 500 universities around the world. Students seeking 21st century skills and top quality education come to Ewha to study in both degree-seeking and short- term programs. Our prominent faculty members, composed of leading scholars and researchers, challenge students to expand their horizons of knowledge and to maximize their intellectual potential. Ewha’s Office of International Affairs continues to play a central role in the university’s international mission, promoting global perspectives among students, faculty and the community.