Geospatial Approach for Assessment of Groundwater Quality
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiruchirappal Disaster Managem Iruchirappalli
Tiruchirappalli District Disaster Management Plan – 2020 THIRUCHIRAPPALLI DISTRICT DISASTER MANAGEMENT PLAN-2020 Tiruchirappalli District Disaster Management Plan – 2020 INDEX S. Particulars Page No. No. 1. Introduction 1 2. District Profile 2-4 3. Disaster Management Goals (2017-2030) 5-11 4. Hazard, Risk and Vulnerability Analysis with Maps 12-49 (District map, Division maps, Taluk maps & list of Vulnerable area) 5. Institutional Mechanism 50-52 6. Preparedness Measures 53-56 7. Prevention and Mitigation measures (2015 – 2030) 57-58 8. Response Plan 59 9. Recovery and Reconstruction Plan 60-61 10. Mainstreaming Disaster Management in Development Plans 62-63 11. Community and other Stake holder participation 64-65 12. Linkages / Co-ordination with other agencies for Disaster Management 66 13. Budget and Other Financial allocation – Outlays of major schemes 67 14. Monitoring and Evaluation 68 15. Risk Communication Strategies 69-70 16. Important Contact Numbers and provision for link to detailed information 71-108 (All Line Department, BDO, EO, VAO’s) 17. Dos and Don’ts during all possible Hazards 109-115 18. Important Government Orders 116-117 19. Linkages with Indian Disaster Resource Network 118 20 Vulnerable Groups details 118 21. Mock Drill Schedules 119 22. Date of approval of DDMP by DDMA 120 23. Annexure 1 – 14 120-148 Tiruchirappalli District Disaster Management Plan – 2020 LIST OF ABBREVIATIONS S. Abbreviation Explanation No. 1. AO Agriculture Officer 2 AF Armed Forces 3 BDO Block Development Officers 4 DDMA District Disaster Management Authority 5 DDMP District Disaster Management Plan 6 DEOC District Emergency Operations Center 7 DRR Disaster Risk Reduction 8 DERAC District Emergency Relief Advisory Committee. -
Executive Summary Book TRICHIRAPALLI.Pmd
THIRUCHIRAPALLI DISTRICT EXECUTIVE SUMMARY DISTRICT HUMAN DEVELOPMENT REPORT TRICHIRAPALLI DISTRICT Introduction The district of Tiruchirappalli was formerly called by the British as ‘Trichinopoly’ and is commonly known as ‘Tiruchirappalli’ in Tamil or Tiruchirappalli‘ in English. The district in its present size was formed in September 1995 by trifurcating the composite Tiruchirappalli district into Tiruchirappalli, Karur and Perambalur districts. The district is basically agrarian; the industrial growth has been supported by the public sector companies like BHEL, HAPP, OFT and Railway workshop. The district is pioneer in fabrication industry and the front runner in the fabrication of windmill towers in the country. As two rivers flow through the district, the Northern part of the district is filled with greeneries than other areas of the district. The river Cauvery irrigates about 51,000 ha. in Tiruchirappalli, Lalgudi and Musiri Divisions. Multifarious crops are grown in this district and Agriculture is the main occupation for most of the people in the District. With an area of 36,246 hectares under the coverage of the forests the district accounts for 1.65 percentage of the total forest area of 1 the State. Honey and Cashewnuts are the main forest produces besides fuel wood. The rivers Kaveri (also called Cauvery) and the river Coleroon (also called Kollidam) flow through the district. There are a few reserve forests along the river Cauvery, located at the west and the north-west of the city. Tiruchirappalli district has been divided into three revenue divisions, viz., Tiruchirappalli, Musiri and Lalgudi. It is further classified into 14 blocks, viz., Andanallur, Lalgudi, Mannachanallur, Manigandam, Manapparai, Marungapuri, Musiri, Pullambadi, Thiruvarumbur, Thottiyam, Thuraiyur, T.Pet, Uppiliyapuram, and Vaiyampatti. -
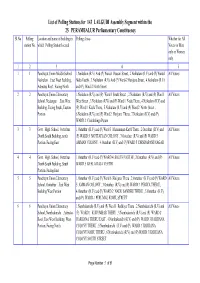
List of Polling Stations for 143 LALGUDI Assembly Segment Within
List of Polling Stations for 143 LALGUDI Assembly Segment within the 25 PERAMBALUR Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 12 3 4 5 1 1 Panchayat Union Middle School, 1.Neikulam (R.V) And (P) Ward-1 Poosari Street , 2.Neikulam (R.V) and (P) Ward-1 All Voters Neikulam ,East West Building, Mela Veethi , 3.Neikulam (R.V) And (P) Ward-2 Harijana Street , 4.Neikulam (R.V) Asbestos Roof , Facing North and (P) Ward-2 North Street 2 2 Panchayat Union Elementary 1.Neikulam (R.V) and (P) Ward 1 South Street , 2.Neikulam (R.V) and (P) Ward 1 All Voters School, Nedungur ,East West West Street , 3.Neikulam (R.V) and (P) Ward 1 Nadu Theru , 4.Neikulam (R.V) and Building, Facing South, Eastern (P) Ward 1 Keela Theru , 5.Neikulam (R.V) and (P) Ward 2 North Street , Portion 6.Neikulam (R.V) and (P) Ward 2 Harijana Theru , 7.Neikulam (R.V) and (P) WARD 2 Chokkalinga Puram 3 3 Govt . High. School, Oottathur 1.Ootathur (R.V) and (P) Ward 3 Mariamman Kovil Theru , 2.Ootathur (R.V) and All Voters ,North South Building, north (P) WARD 5 MOTTAIYAN COLONY , 3.Ootathur (R.V) and (P) WARD 5 Portion, Facing East AMMAN COLONY , 4.Ootathur (R.V) and (P) WARD 5 DHNDAPANI NAGAR 4 4 Govt . High. School, Oottathur 1.Ootathur (R.V) and (P) WARD 4 SOUTH VEETHI , 2.Ootathur (R.V) and (P) All Voters ,North South Building, South WARD 5 KEELA RAJA VEETHI Portion, Facing East 5 5 Panchayat Union Elementary 1.Ootathur (R.V) and (P) Ward 6 -

Ii Pullambadi Canal
DEPARTMENT OF ECONOMICS St. JOSEPH’S COLLEGE (Autonomous) (Affiliated to Bharathidasan University, Tiruchirappalli) TIRUCHIRAPPALLI – 620 002. Dr. G. GNANASEKARAN M.A., M.B.A., M.Phil., Ph.D., Head & Research Advisor. CERTIFICATE This is to certify that the thesis entitled “AN ECONOMIC ANALYSIS OF WATER USE EFFICIENCY OF FARMERS IN PULLAMBADI CANAL OF TIRUCHIRAPPALLI AND ARIYALUR DISTRICTS, TAMIL NADU” submitted by Mr. G. IRUTHAYARAJ (Reg. No. 011148 / Ph.D.2 / Economics / F.T. / July 2007) is a bonafide record of research work done by him under my guidance as a full time scholar in the Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli and that the thesis has not previously formed the basis for the award to the candidate of any degree or any other similar title. The thesis is the outcome of personal research work done by the candidate under my overall supervision. (G. GNANASEKARAN) Station: Tiruchirappalli Date : DECLARATION I hereby declare that the work embodied in this thesis has been originally carried out by me under the guidance and supervision of Dr. G. GNANASEKARAN , Head and Research Advisor, Department of Economics, St. Joseph’s College (Autonomous), Tiruchirappalli - 620 002. This work has not been submitted either in full or in part for any other degree or diploma at any university. (G. IRUTHAYARAJ) Research Scholar Place: Tiruchirappalli Date : ACKNOWLEDGEMENT I wish to place on record the valuable help rendered by various people to complete this dissertation work. I would like to express my profound sense of gratitude to my research adviser and Best Teacher Awardees Dr. G. Gnanasekaran M.A., M.B.A., M.Phil., Ph.D., Head and Associate Professor of Economics, for his stimulating guidance by spending his valuable time with me in sharpening my thinking and analysis, valuable suggestions and continuous encouragement throughout the study. -

I. Profile of Pudukkottai District
I. PROFILE OF PUDUKKOTTAI DISTRICT 1. INTRODUCTION Pudukkottai has a familiar Historical background and it was formerly a Princely State with the title of “SAMASTHANAM” ruled by the “H.H.The Rajah’s of THONDAIMANS”. The present Pudukkottai district is encompassing the entire Princely State of Pudukkottai and parts of Tiruchirappalli and Thanjavur districts. Pudukkottai district came into existence on 14.1.1974. The erstwhile “Pudukkottai State” has been justly famous for its efficient and stable administration through the years with its seasoned administrative system, operating with well understood concepts of hierarchy line of command and discreet adherences to principles and procedures. Really this credit goes to the initial author and as well as the founder of the system of “District Office Manual”, by “Sir Alexander Loftus Tottenham”, the Agent of the British Emperor/ Administrator of erstwhile “Pudukkottai State” for his aim of trim and efficient administration. Pudukkotai District is bounded on the North East and East by Thanjavur District, on the South East by Bay of Bengal, on the South West by Ramanathapuram and Sivaganga districts and on the West and North East by Thiruchirapalli District. 1 2. DISASTER MANAGEMENT PLAN The main objective of Disaster Management Plan is to assess the vulnerability of district to various major hazards so that mitigate steps can be taken to contain the damages before and during disaster and to provide relief and take reconstruction measures at the shortest possible time effectively. The District Disaster Management Plan is also a purposeful document that assigns responsibility to the officials of Government Departments, Social Organisations and Individuals for carrying out specific and effective actions at projected times and places in an emergency manner that exceeds the capability or routine responsibility of an one agency, e.g 2 the departments of Revenue, Police, Fire Services, Fisheries, Highways, PWD, South Vellar Division and Health etc. -
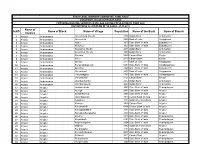
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

Chapter 4.1.9 Ground Water Resources Trichy District
CHAPTER 4.1.9 GROUND WATER RESOURCES TRICHY DISTRICT 1 INDEX CHAPTER PAGE NO. INTRODUCTION 3 TRICHY DISTRICT – ADMINISTRATIVE SETUP 3 1. HYDROGEOLOGY 3-7 2. GROUND WATER REGIME MONITORING 8-15 3. DYNAMIC GROUND WATER RESOURCES 15-24 4. GROUND WATER QUALITY ISSUES 24-25 5. GROUND WATER ISSUES AND CHALLENGES 25-26 6. GROUND WATER MANAGEMENT AND REGULATION 26-32 7. TOOLS AND METHODS 32-33 8. PERFORMANCE INDICATORS 33-36 9. REFORMS UNDERTAKEN/ BEING UNDERTAKEN / PROPOSED IF ANY 10. ROAD MAPS OF ACTIVITIES/TASKS PROPOSED FOR BETTER GOVERNANCE WITH TIMELINES AND AGENCIES RESPONSIBLE FOR EACH ACTIVITY 2 GROUND WATER REPORT OF TRICHY DISTRICT INRODUCTION : In Tamil Nadu, the surface water resources are fully utilized by various stake holders. The demand of water is increasing day by day. So, groundwater resources play a vital role for additional demand by farmers and Industries and domestic usage leads to rapid development of groundwater. About 63% of available groundwater resources are now being used. However, the development is not uniform all over the State, and in certain districts of Tamil Nadu, intensive groundwater development had led to declining water levels, increasing trend of Over Exploited and Critical Firkas, saline water intrusion, etc. ADMINISTRATIVE SET UP The Geographical area of Tiruchirappalli district is 4, 40,383 hectares (4403.83 sq.km) accounting for 3.38 percent of geographical area of Tamil Nadu State. The district has well laid out roads and railway lines connecting all major towns within and outside the state. For administrative purpose, this district has been bifurcated into 8 Taluks, 14 Blocks and 41 Firkas. -

District Industrial Profile Trichy
Government of India Ministry of MSME District Industrial Profile Trichy 2019-20 Prepared by M S M E - D e v e l o p m e n t I n s t i t u t e, C h e n n a i (Ministry of MSME, Govt. of India,) 65/1, MSME Bhawan, GST Road, Guindy, Chennai, Tamil Nadu - 600032 Phone Tel: +91 44-22501011, 12, 13, Fax: +91 44-22501014 E-mail: [email protected] Website:- www.dcmsme.gov.in / www.msmedi-chennai.gov.in CONTENTS CHAPTER NO. TITLE PAGE NO. 1 TIRUCHIRAPPALLI DISTRICT AT A GLANCE 1 2 SALIENT FEATURES OF THE DISTRICT 10 3 RESOURCES AVAILABLE IN THE DISTRICT 13 4 INFRASTRUCTURE FACILITIES IN THE DISTRICT 18 5 INDUSTRIAL SCENARIO IN THE DISTRICT 26 6 STEPS TO START MSME ENTERPRISES 54 7 GOVERNMENT SCHEMES FOR ENTREPRENEURS 55 8 CONTACT ADDRESSES FOR ENTREPRENEURS 58 LIST OF TABLES TABLE NO. TITLE PAGE NO. TABLE 1.1 IMPORTANT STATISTICS OF THE DISTRICT 1 TABLE 1.2 VITAL STATISTICS OF THE DISTRICT 4 TABLE 1.3 RAINFALL IN THE DISTRICT 4 TABLE 1.4 ADMINISTRATIVE SET UP OF THE DISTRICT 5 TABLE 3.1 LAND CLASSIFICATION AND UTILISATION 13 TABLE 3.2 CULTIVATION AREA, MAJOR CROPS AND PRODUCTION 14 TABLE 3.3 PLACES OF INTEREST FOR TOURISM 17 TABLE 4.1 NATIONAL HIGHWAYS PASSING THROUGH THE DISTRICT 19 TABLE 4.2 PASSENGER AND CARGO MOVEMENTS FROM AIRPORT 19 TABLE 4.3 SECTOR WISE POWER CONSUMPTION IN THE DISTRICT 20 TABLE 4.4 PERFORMANCE OF COMMERCIAL BANKS IN THE DISTRICT 22 TABLE 4.5 NUMBER OF BANK BRANCHES IN THE DISTRICT 23 TABLE 5.1 DEFINITIONS OF MSME ENTERPRISES 26 TABLE 5.2 NUMBER OF MSMEs IN THE DISTRICT 28 TABLE 5.3 INVESTMENT IN MSMEs IN THE DISTRICT -

Tiruchirappalli District
CENSUS OF INDIA 2011 TOTAL POPULATION AND POPULATION OF SCHEDULED CASTES AND SCHEDULED TRIBES FOR VILLAGE PANCHAYATS AND PANCHAYAT UNIONS TIRUCHIRAPPALLI DISTRICT DIRECTORATE OF CENSUS OPERATIONS TAMILNADU ABSTRACT TIRUCHIRAPPALLI DISTRICT No. of Total Total Sl. No. Panchayat Union Total Male Total SC SC Male SC Female Total ST ST Male ST Female Village Population Female 1 Andanallur 25 89,225 44,677 44,548 23,937 11,774 12,163 30 17 13 2 Manikandam 22 1,07,526 53,312 54,214 18,986 9,275 9,711 498 247 251 3 Thiruverambur 20 1,05,191 52,713 52,478 21,696 10,863 10,833 491 259 232 4 Manapparai 21 1,07,837 53,942 53,895 19,187 9,610 9,577 151 93 58 5 Marungapuri 49 1,18,370 58,929 59,441 25,169 12,557 12,612 - - - 6 Vaiyampatti 18 96,463 47,844 48,619 14,956 7,345 7,611 10 5 5 7 Lalgudi 45 1,19,238 58,674 60,564 31,344 15,202 16,142 463 249 214 8 Manachanallur 35 1,53,865 76,964 76,901 29,077 14,366 14,711 159 89 70 9 Pullambadi 33 82,137 40,208 41,929 15,054 7,458 7,596 148 79 69 10 Musiri 33 1,00,879 50,147 50,732 23,894 11,682 12,212 41 25 16 11 Thottiam 26 1,09,278 54,483 54,795 22,226 10,941 11,285 10 4 6 12 Tattayyangarpettai 25 81,388 41,188 40,200 14,501 7,240 7,261 115 70 45 13 Thuraiyur 34 1,13,343 56,276 57,067 23,735 11,730 12,005 7,076 3,674 3,402 14 Uppiliyapuram 18 87,205 43,025 44,180 21,347 10,457 10,890 5,327 2,722 2,605 Grand Total 404 14,71,945 7,32,382 7,39,563 3,05,109 1,50,500 1,54,609 14,519 7,533 6,986 ANDANALLUR PANCHAYAT UNION Sl. -

Tiruchirappalli
TIRUCHIRAPPALLI S.No. ROLL No. NAME OF ADVOCATE ADDRESS 3/48, KOTTA KOLLAI STREET, BEEMA NAGAR, 1 1911/2013 ABDUL HAKEEM A. TIRUCHI 620001 53-ALLWARTHOPE STREET, PALAKKARAI, 2 12/1971 ABDUL MALIK Y.K. TRICHI - 8. 45/1, R.K. GARDEN AKILANDESWARI NAGAR, 3 124/1983 ABRAHAM PREMKUMAR P. LALGUDI - 621601, TRICHY. NO. 38, CAVERY NAGAR, SRIRANGAM, 4 1004/2007 ADHINARAYANAMOORTHY R. TRICHY - 620 006 NO. 57 MAIN ROAD, THIRUVALAR SOLAI 5 2142/2007 ADITHAN S POST, THIRUVANAI KOVIL VIA, SRIRANGAM TALUK, TRICHY DISTRICT - 620 005 84A, PUKKATHURAI POST, MANACHANALLUR 6 2543/2007 AGILAN S. TK. TRICHY DT. 621213. B/3, HOUSING UNIT, VARAGANERY COLONY, 7 3002/2012 AGILESVARAA T.K. TANJAVUR ROAD, TRICHY - 8. 3-B, BALAJI BLOCK, S.B.O. COLONY, 8 1159/1996 AGNEL RAJAN A. CANTONMENT, TIRUCHIRAPPALLI -620001 3D, ROYAL FANTASY FLATS, STATE BANK 9 83/1990 AHAMATH BATHUSHA A. OFFICERS COLONY, LAWYERS ROAD, TRICHY - 1 NO.74, VARUSAI ROWTHER LANE, TANJORE 10 839/1995 AHMED BASHA S. ROAD, TRICHY-620008. 3/108A, OLD POST OFFICE STREET, 11 471/1999 AJMAL KAN A. INAMKULATHUR P.O. TRICHY DT. S.No. ROLL No. NAME OF ADVOCATE ADDRESS 7, RAMA RAO ST., TENNUR HIGH ROAD, 12 638/1986 AJUHAR ALI A. TRICHIRAPPALLI - 620 017 94, SRIRAMAPURAM, RAYAR THOPE, 13 961/1998 AKILA S. SRIRANGAM, TRICHY 620006. 1/97, MAIN ROAD, MANAKKAL POST, LALGUDI 14 1355/2014 AKILANDESWARI A. TALUK, TRICHY - 621 601 NO.41, MALLIGAIPURAM MAIN ROAD, 15 42/2015 ALAGAPPAN A. MALLIGAIPURAM, PALAKARAI, TRICHY- 620001. 43/44-B, MUTHURAJA STREET, 16 2108/2006 ALAGAR M. INAMSAMYAPURAM, MANNACHANALLUR, TRICHY 621112. -
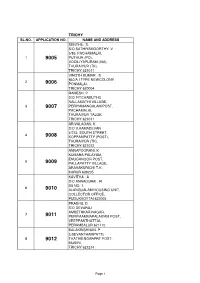
Trichy Sl.No
TRICHY SL.NO. APPLICATION NO. NAME AND ADDRESS SENTHIL. S. S/O SATHIYAMOORTHY. V 3/85, PACHAIMALAI, 1 9005 PUTHUR (PO), VOOILIYAPURAM (VIA), THURAIYUR (TK), TRICHY 621011 VINOTH KUMAR . S 930/A I TYPE NEWCOLONY, 2 9006 PONMALAI, TRICHY 620004 RAMESH. P S/O PITCHAMUTHU NALLAMATHI VILLAGE, 3 9007 PERIYAMANGALAM POST, PACHAMALAI, THURAIYUR TALUK, TRICHY 621011 ARIVALAGAN. K S/O V.KAMADEVAN 4/273, SOUTH STREET, 4 9008 KOPPAMPATTY (POST), THURAIYUR (TK), TRICHY 621012 ANNAPOORANI. K KUMARA PALAYAM, ENUGANOOR POST, 5 9009 PALLAPATTY VILLAGE, ARAVAKURICHI T.K , KARUR 639205 KAVITHA . A S/O ANNADURAI . R D5 NO. 1, 6 9010 ALANGUALAM HOUSING UNIT, COLLECTOR OFFICE, PUDUKKOTTAI 622005 PRABHU. D S/O DEVARAJ AMBETHKAR NAGAR, 7 9011 PERIYAAMMAPALAIYAM POST, VEEPPANTHATTAI, PERAMBALUR 621110 BALAKRISHNAN. P 3,SEVANTHAMPATTI, 8 9012 THATHIENGARPRT POST, MUSIRI, TRICHY 621214 Page 1 SUSEELA . M 75/1 GANESAPURAM 9 9013 NEW STREET PONMALAI TRICHY 620004 MADHAVAN.D 9/133A, PERIYAR STREET, 10 9014 THUVAKUDI MALAI (SOUTH), M.D. SALAI, TRICHY 620022 VELMURUGAN. A 13/19D-11K3, 11 9015 K.K.NAGAR EXTENSION, RAJAJI NAGAR (PO), ARIYALUR 621713 MURALI THARAN. S S/O SRINIVASAN. A NO.9, KAMARAJAR STREET, 12 9016 VIVEGANANTHA NAGAR, MELA KALKANDAR KOTTAI, TRICHY 620011 THIRUMURUGAN. K S/O KANNAPPAN. A 1/53, MAIN ROAD, 13 9017 PAPPAKKUDI POST, MEENSURUTTI VIA, UDAYARPALAYAM TALUK, ARIYALUR 612903 SATHISH. S S/O SOLAI. K 14 9018 91/A AMBETHKAR NAGAR, ALANGUDI PO & TALUK, PUDUKOTTAI 622301 SARAVANAKUMAR .S S/O SHANMUGAM 43, 6TH CROSS STREET, 15 9019 PARVATHI PURAM, MUSIRI TK, TRICHY 621211 SATHEESH.J S/O JAYAPAL K.K. NAGAR, 16 9020 KRISHNAPURAM PO, VEPPANTHATTAI (TK), PERAMBALUR 621116 Page 2 MOHAN.P S/O PERIYASAMY N.P ELANGO STREET, 17 9021 NAGAIYANALLUR POST, KATTUPUTHUR (VIA), THOTTIYAM (TK), TRICHY 621207 UMA MAHESWARI. -
Assesment of Groundwater Pollution in and Around Lalgudi Taluk, Trichy District, Tamilnadu, India
AEGAEUM JOURNAL ISSN NO: 0776-3808 ASSESMENT OF GROUNDWATER POLLUTION IN AND AROUND LALGUDI TALUK, TRICHY DISTRICT, TAMILNADU, INDIA R. Arulnangai, M. Mohamed Sihabudeeen & S. Farook Basha PG and Research Department of Chemistry, Jamal Mohamed College (Autonomous), Tiruchirapalli - 20 (Affiliated to Bharathidasan University) Tiruchirapalli - 20 ______________________________________________________________________________ Abstract Physicochemical and Heavy metal analysis was carried out in Lalgudi taluk at Tiruchirapalli District in Tamilnadu with the objective of understanding the suitability of groundwater quality for domestic and irrigation purposes. Ground water samples were collected from 8 locations during (March 2020) analyzed for physico - chemical parameters such as pH, Electrical Conductivity, Total Dissolved Solids, Total Hardness, Calcium, Magnesium, Carbonate, Bicarbonate, Chloride, Dissolved oxygen, Biological oxygen demand, Chemical oxygen demand, Copper, Zinc and Iron. In the present study calculated the value of ground water of Tiruchirapalli District. In this study water quality clearly shows that the status of the water body is unsuitable for drinking purpose. Keywords: Groundwater, physico – chemical parameters and Heavy metal analysis, Lalgudi taluk Volume 8, Issue 7, 2020 http://aegaeum.com/ Page No:1012 AEGAEUM JOURNAL ISSN NO: 0776-3808 INTRODUCTION Water is the basis of all life. It is fundamental for human existence, ecological balance and for the very future of our planet. Water covers about 70% Humans and other animals have developed senses that enable them to evaluate the portability of water by avoiding water that is too salty or putrid. Water plays an important role in the world economy. (1)Approximately 70% of the freshwater used by humans goes to agriculture. Fishing in salt and fresh water bodies is a major source of food for many parts of the world.