Long-Term Movements and Activity Patterns of Platypus on Regulated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Assessment of Thermal Pollution in the Snowy River Below Jindabyne
Assessment of thermal pollution Snowy River Increased Flows Assessment of thermal pollution in the Snowy River below Jindabyne Dam Summary This study assessed water temperature in the highly regulated Snowy River below Jindabyne Dam. What did we study? • The thermal regime of rivers plays a critical role in the health of aquatic ecosystems. We monitored water temperature below Jindabyne Dam to assess the thermal regime of the regulated Snowy River. Monitoring began in 2011, following the installation of a multi-level offtake and the delivery of environmental flows. It continued until 2013. During this time we compared the regulated thermal regime with a natural thermal regime model. The model was developed using pre-dam (1962-1967) water temperature data. What did we find? • Cold water pollution in the Snowy River below Jindabyne Dam is not a significant issue. • Warm water pollution remains an issue in the regulated Snowy River, particularly between March and July. Yet water temperatures are rarely more than 2 - 4°C warmer than natural. Temperatures never exceed the expected natural temperature range of the Snowy River before Jindabyne Dam. • Water temperatures match the expected natural water temperatures at the town of Dalgety. This is approximately 20km downstream of Jindabyne Dam. What does this mean for water management? • The multi-level offtake has improved the thermal regime in this regulated river. Additional management strategies to improve the thermal regime are limited. An alternate release point in the Mowamba River may further improve water temperatures. The extent of improvements would depend on the dilution of flow volumes from the Mowamba River into the Snowy River. -
Gauging Station Index
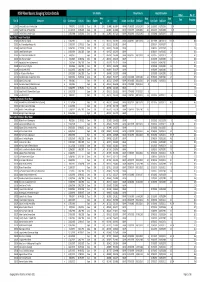
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

Rivers and Streams Special Investigation Final Recommendations
LAND CONSERVATION COUNCIL RIVERS AND STREAMS SPECIAL INVESTIGATION FINAL RECOMMENDATIONS June 1991 This text is a facsimile of the former Land Conservation Council’s Rivers and Streams Special Investigation Final Recommendations. It has been edited to incorporate Government decisions on the recommendations made by Order in Council dated 7 July 1992, and subsequent formal amendments. Added text is shown underlined; deleted text is shown struck through. Annotations [in brackets] explain the origins of the changes. MEMBERS OF THE LAND CONSERVATION COUNCIL D.H.F. Scott, B.A. (Chairman) R.W. Campbell, B.Vet.Sc., M.B.A.; Director - Natural Resource Systems, Department of Conservation and Environment (Deputy Chairman) D.M. Calder, M.Sc., Ph.D., M.I.Biol. W.A. Chamley, B.Sc., D.Phil.; Director - Fisheries Management, Department of Conservation and Environment S.M. Ferguson, M.B.E. M.D.A. Gregson, E.D., M.A.F., Aus.I.M.M.; General Manager - Minerals, Department of Manufacturing and Industry Development A.E.K. Hingston, B.Behav.Sc., M.Env.Stud., Cert.Hort. P. Jerome, B.A., Dip.T.R.P., M.A.; Director - Regional Planning, Department of Planning and Housing M.N. Kinsella, B.Ag.Sc., M.Sci., F.A.I.A.S.; Manager - Quarantine and Inspection Services, Department of Agriculture K.J. Langford, B.Eng.(Ag)., Ph.D , General Manager - Rural Water Commission R.D. Malcolmson, M.B.E., B.Sc., F.A.I.M., M.I.P.M.A., M.Inst.P., M.A.I.P. D.S. Saunders, B.Agr.Sc., M.A.I.A.S.; Director - National Parks and Public Land, Department of Conservation and Environment K.J. -

The Geology and Prospectivity of the Tallangatta 1:250 000 Sheet
VIMP Report 10 The geology and prospectivity of the Tallangatta 1:250 000 sheet I.D. Oppy, R.A. Cayley & J. Caluzzi November 1995 Bibliographic reference: OPPY, I.D., CAYLEY R.A. & CALUZZI, J., 1995. The Geology and prospectivity of the Tallangatta 1:250 000 sheet Victorian Initiative for Minerals and Petroleum Report 10. Department of Agriculture, Energy and Minerals. © Crown (State of Victoria) Copyright 1995 Geological Survey of Victoria ISSN 1323 4536 ISBN 0 7306 7980 2 This report may be purchased from: Business Centre, Department of Agriculture, Energy & Minerals, Ground Floor, 115 Victoria Parade, Fitzroy, Victoria 3065 For further technical information contact: General Manager, Geological Survey of Victoria, Department of Agriculture, Energy & Minerals, P O Box 2145, MDC Fitzroy, Victoria 3065 Acknowledgments: The authors wish to acknowledge G. Ellis for formatting the document, R. Buckley, P.J. O'Shea and D.H. Taylor for editing and S. Heeps for cartography I. Oppy wrote chapters 3 and 5, R. Cayley wrote chapter 2 and J. Caluzzi wrote chapter 4. GEOLOGY AND PROSPECTIVITY - TALLANGATTA 1 Contents Abstract 4 1 Introduction 5 2 Geology 7 2.1 Geological history 7 Pre-Ordovician to Early Silurian 7 Early Silurian Benambran deformation and widespread granite intrusion 8 Middle to Late Silurian 9 Late Silurian Bindian deformation 9 Early Devonian rifting and volcanism 10 Middle Devonian Tabberabberan deformation 11 Late Devonian sedimentation and volcanism 11 Early Carboniferous Kanimblan deformation to Present day 11 2.2 Stratigraphy -

Government Gazette of the STATE of NEW SOUTH WALES Number 112 Monday, 3 September 2007 Published Under Authority by Government Advertising
6835 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 112 Monday, 3 September 2007 Published under authority by Government Advertising SPECIAL SUPPLEMENT EXOTIC DISEASES OF ANIMALS ACT 1991 ORDER - Section 15 Declaration of Restricted Areas – Hunter Valley and Tamworth I, IAN JAMES ROTH, Deputy Chief Veterinary Offi cer, with the powers the Minister has delegated to me under section 67 of the Exotic Diseases of Animals Act 1991 (“the Act”) and pursuant to section 15 of the Act: 1. revoke each of the orders declared under section 15 of the Act that are listed in Schedule 1 below (“the Orders”); 2. declare the area specifi ed in Schedule 2 to be a restricted area; and 3. declare that the classes of animals, animal products, fodder, fi ttings or vehicles to which this order applies are those described in Schedule 3. SCHEDULE 1 Title of Order Date of Order Declaration of Restricted Area – Moonbi 27 August 2007 Declaration of Restricted Area – Woonooka Road Moonbi 29 August 2007 Declaration of Restricted Area – Anambah 29 August 2007 Declaration of Restricted Area – Muswellbrook 29 August 2007 Declaration of Restricted Area – Aberdeen 29 August 2007 Declaration of Restricted Area – East Maitland 29 August 2007 Declaration of Restricted Area – Timbumburi 29 August 2007 Declaration of Restricted Area – McCullys Gap 30 August 2007 Declaration of Restricted Area – Bunnan 31 August 2007 Declaration of Restricted Area - Gloucester 31 August 2007 Declaration of Restricted Area – Eagleton 29 August 2007 SCHEDULE 2 The area shown in the map below and within the local government areas administered by the following councils: Cessnock City Council Dungog Shire Council Gloucester Shire Council Great Lakes Council Liverpool Plains Shire Council 6836 SPECIAL SUPPLEMENT 3 September 2007 Maitland City Council Muswellbrook Shire Council Newcastle City Council Port Stephens Council Singleton Shire Council Tamworth City Council Upper Hunter Shire Council NEW SOUTH WALES GOVERNMENT GAZETTE No. -

Heritage Rivers Act 1992 No
Version No. 014 Heritage Rivers Act 1992 No. 36 of 1992 Version incorporating amendments as at 7 December 2007 TABLE OF PROVISIONS Section Page 1 Purpose 1 2 Commencement 1 3 Definitions 1 4 Crown to be bound 4 5 Heritage river areas 4 6 Natural catchment areas 4 7 Powers and duties of managing authorities 4 8 Management plans 5 8A Disallowance of management plan or part of a management plan 7 8B Effect of disallowance of management plan or part of a management plan 8 8C Notice of disallowance of management plan or part of a management plan 8 9 Contents of management plans 8 10 Land and water uses which are not permitted in heritage river areas 8 11 Specific land and water uses for particular heritage river areas 9 12 Land and water uses which are not permitted in natural catchment areas 9 13 Specific land and water uses for particular natural catchment areas 10 14 Public land in a heritage river area or natural catchment area is not to be disposed of 11 15 Act to prevail over inconsistent provisions 11 16 Managing authority may act in an emergency 11 17 Power to enter into agreements 12 18 Regulations 12 19–21 Repealed 13 22 Transitional provision 13 23 Further transitional and savings provisions 14 __________________ i Section Page SCHEDULES 15 SCHEDULE 1—Heritage River Areas 15 SCHEDULE 2—Natural Catchment Areas 21 SCHEDULE 3—Restricted Land and Water Uses in Heritage River Areas 25 SCHEDULE 4—Specific Land and Water Uses for Particular Heritage River Areas 27 SCHEDULE 5—Specific Land and Water Uses for Particular Natural Catchment Areas 30 ═══════════════ ENDNOTES 31 1. -

Sydneyœsouth Coast Region Irrigation Profile
SydneyœSouth Coast Region Irrigation Profile compiled by Meredith Hope and John O‘Connor, for the W ater Use Efficiency Advisory Unit, Dubbo The Water Use Efficiency Advisory Unit is a NSW Government joint initiative between NSW Agriculture and the Department of Sustainable Natural Resources. © The State of New South Wales NSW Agriculture (2001) This Irrigation Profile is one of a series for New South Wales catchments and regions. It was written and compiled by Meredith Hope, NSW Agriculture, for the Water Use Efficiency Advisory Unit, 37 Carrington Street, Dubbo, NSW, 2830, with assistance from John O'Connor (Resource Management Officer, Sydney-South Coast, NSW Agriculture). ISBN 0 7347 1335 5 (individual) ISBN 0 7347 1372 X (series) (This reprint issued May 2003. First issued on the Internet in October 2001. Issued a second time on cd and on the Internet in November 2003) Disclaimer: This document has been prepared by the author for NSW Agriculture, for and on behalf of the State of New South Wales, in good faith on the basis of available information. While the information contained in the document has been formulated with all due care, the users of the document must obtain their own advice and conduct their own investigations and assessments of any proposals they are considering, in the light of their own individual circumstances. The document is made available on the understanding that the State of New South Wales, the author and the publisher, their respective servants and agents accept no responsibility for any person, acting on, or relying on, or upon any opinion, advice, representation, statement of information whether expressed or implied in the document, and disclaim all liability for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information contained in the document or by reason of any error, omission, defect or mis-statement (whether such error, omission or mis-statement is caused by or arises from negligence, lack of care or otherwise). -

Fire Operations Plan
o! O l d y m R p a l l i c a H U W w r a a l l J n y a i a n W g R d e d - R l l a a i r c om e R o d T d n i J FINAL J i S n H o g u e u t ll m h ic d R e R d a H n U w r w o a B n y Fire Operations Plan a Rd S iver t R UPPER MURRAY THOLOGOLONG - KURRAJONG WALWA- Walwa M y TRK SNAKE urray R Wym ra iver R ah Rd r r d u e GULLY g v an M i eg R lar DISTRICT e side Rd W den Ar Rd d R g U n r a a g n e d r a a R Pine l R # # # e d a Mountain RA W New South g g # # # a Wales W # # # # # # Ri MT LAWSON SP - E 2014-2015 TO 2016-2017 ve # # # Pine ri na MOTHER # # # H Burrowye Mountain wy DUFF Tinta Bungil RA ldra Rd MT KELLY # # t # # # TINTALDRA S e t ALBURY # # # - JEREMAL a Bungil M # # # # # # NCR HEAPS AIRPORT d WISES # # # # # R t Lake a Jemba RA S lw o! # # # # # # a Tintaldra g CREEK W n Hume - u # # # y o FLORA RESERVE lle Y # # # # # # # # # # # # # # # # # # # # # # he # # # # PINE S THOLOGOLONG # # # # # # # # # # # # # # # # # # # # # # # # # Map Legend t MTN - S WODONGA - BUNGIL # # # # # # # # # # # # # # # # # # # # # # # # # # SANDY CREEK RD s M n u MCFARLANES y i REFERENCE AREA # # # # # # # # # # # # # # # # # # # # # # # # # rra w k y s t # # # # # # # # # # # # # # # # # # # # # # # # # # R A iv HILL C er ln # # # # # # # # # B#URR# OW#YE # # # # # # # # # # # # # R d d d Transportation H o t R c R S u n # # # # # # # # #- CH#ICK#S TR#ACK# # # # # # # # # # # # # # ra a CUDGEWA m i y d y l e L a Bellbridge a F # r n # # # # # # # # # # # # # # # # # # # # # # # # # t wy y r MT LAWSON - Mt Burrowa a - MCNAMARAS TRK in # # -

31 January 2006 Mowamba Aqueduct Recommissioned the Mowamba
Date: 31 January 2006 Subject: Mowamba Aqueduct Recommissioned The Mowamba Aqueduct has been recommissioned concluding the temporary arrangement of water borrowing to the Snowy River via the Mowamba Weir. The recommissioning enables the 3 kms of the Snowy River closest to the dam to now receive the volume of environmental flows that was intended and described in the outcome of the environmental studies conducted during the Snowy Water Inquiry. The recommissioning of the aqueduct was undertaken in accordance with the provisions of the Snowy Water Licence. Those provisions are as agreed between the Commonwealth, New South Wales and Victorian Governments on corporatisation of the Snowy Mountains Scheme in June 2002. It now means that environmental flows to the Snowy River will be fully provided from Jindabyne Dam, as agreed to by the New South Wales, Victorian and Commonwealth Governments and as Snowy Hydro Limited has been directed. Those flows are now occurring. Snowy Hydro Limited Managing Director, Mr Terry Charlton, said: “The recommissioning of the aqueduct means that the Snowy River immediately downstream of Jindabyne Dam is receiving a higher volume of environmental flows. This is the outcome we all wanted.” “Further downstream at Dalgety and beyond, the environmental flow in the Snowy River is the same as the Snowy River was receiving before the recommissioning - and of course we will continue to additionally maintain a riparian flow down the Mowamba River below the Weir.” “Snowy Hydro has undertaken this work as part of our obligations under the Snowy Water Licence.” “Transferring an amount of water from the Mowamba River into Lake Jindabyne and then into the Snowy River is integral and critical to the water release arrangements that required the company to spend over $90 million upgrading and modifying the Jindabyne Dam, including works that enable us to release only oxygenated water from the top surface of the dam. -

Concept Plan
Monaro Acclimatisation Society Inc 9 Thompson Drive Tathra, NSW. 2550 Sustainable future fishing for trout and native fish SNOWY HYDRO 2.0 – MAS CONCEPT PLAN On 2 July 2020 MAS Secretary Rod Whiteway and I met with senior officers from NSW Fisheries to discuss the MAS Concept Plan for a trout grow-out facility as a recreational fishing offset for Snowy Hydro 2.0. Following that meeting NSW Fisheries have asked the MAS to formally enter into “Agreed Principles” arrangement. The MAS Executive is currently discussing this Agreement; however, we indicated we have four key priorities that we are committed to. On 7 July 2020 I met with senior staff from Snowy Hydro to discuss the MAS Concept Plan. Again, we explained our key priorities and Snowy Hydro did not express any negativity to our approach. Snowy Hydro indicated they are keen for a meeting between MAS, DPI and Snowy Hydro in the not too distant future to begin working formally on the plan. THE OVERALL CONCEPT Since the announcement of Snowy Hydro 2.0 the MAS has been concerned that redfin perch would be pumped from Talbingo Dam into Tantangara Dam and beyond. To help protect the Snowy Mountains trout fishery the MAS embarked on a process to identify how larger trout could be stocked to counter the expected redfin predation and to protect our trout fishing legacy and reassure the Snowy trout community that something was being done. The MAS soon realised that it was undertaking this process in a policy vacuum, so the MAS began negotiating directly with Snowy Hydro on mitigation measures. -

EIS 1483 AA0681 11 Water Quality in the Snowy River Catchment Area
EIS 1483 AA0681 11 Water quality in the Snowy River catchment area : report on 1996/97 data; nutrient loads in the Thredbo river; trend assessment NSW YEPT PRIMARY IRDUSIRIES I AA0681 11 I LAND &WATER CONSERVATION I I I I I I d I I I I I I I I NSW Department of Land and Water Conservation I I I DEPARTMENT OF LAND & WATER CONSERVATION CENTRE FOR NATURAL RESOURCES I I I WATER QUALITY IN THE SNOWY I RIVER CATCHMENT AREA I - Report on 1996/97 Data - Nutrient Loads in the Thredbo River I - Trend Assessment I I I I I I I I H I I DEPARTMENT OF LAND & WATER CONSERVATION CENTRE FOR NATURAL RESOURCES WATER QUALITY IN THE SNOWY RIVER CATCHMENT AREA - Report on 1996/97 Data - Nutrient Loads in the Thredbo River - Trend Assessment Zenita Acaba, Lee Bowling, Lloyd Flack June 1998 and Hugh Jones CNR 99.005 [s9697co2.Doc] CENTRE FOR NATURAL RESOURCES © Department of Land & Water Conservation ISBN 0 7347 5023 4 Public Document Water Quality in the Snowy River Gatchinent Area, 1996197 Report 1 I I I I I I Cologne I In KOln, a town of monks and bones, I and pavements fang'd with murderous stones I and rags, and hags, and hideous wenches; I counted two and seventy stenches, I All well defined, and several stinks! I Ye Nymphs that reign o'er sewers and sinks, The river Rhine, it is well known, I Doth wash your city of Cologne; But tell me, Nymphs, what power divine I Shall henceforth wash the river Rhine? Li I Samuel Taylor Coleridge, 1828 I I I 1 Water Quality in the Snowy River Catchment Area, 1996/97 Report I I I ACKNOWLEDGEMENTS The majority of the sampling for this project was undertaken by staff of the Snowy Mountains Hydro-Electric Authority's Hydrographic Office at Jindabyne, chiefly by Messrs. -

NSW Recreational Freshwater Fishing Guide 2020-21
NSW Recreational Freshwater Fishing Guide 2020–21 www.dpi.nsw.gov.au Report illegal fishing 1800 043 536 Check out the app:FishSmart NSW DPI has created an app Some data on this site is sourced from the Bureau of Meteorology. that provides recreational fishers with 24/7 access to essential information they need to know to fish in NSW, such as: ▢ a pictorial guide of common recreational species, bag & size limits, closed seasons and fishing gear rules ▢ record and keep your own catch log and opt to have your best fish pictures selected to feature in our in-app gallery ▢ real-time maps to locate nearest FADs (Fish Aggregation Devices), artificial reefs, Recreational Fishing Havens and Marine Park Zones ▢ DPI contact for reporting illegal fishing, fish kills, ▢ local weather, tide, moon phase and barometric pressure to help choose best time to fish pest species etc. and local Fisheries Offices ▢ guides on spearfishing, fishing safely, trout fishing, regional fishing ▢ DPI Facebook news. Welcome to FishSmart! See your location in Store all your Contact Fisheries – relation to FADs, Check the bag and size See featured fishing catches in your very Report illegal Marine Park Zones, limits for popular species photos RFHs & more own Catch Log fishing & more Contents i ■ NSW Recreational Fishing Fee . 1 ■ Where do my fishing fees go? .. 3 ■ Working with fishers . 7 ■ Fish hatcheries and fish stocking . 9 ■ Responsible fishing . 11 ■ Angler access . 14 ■ Converting fish lengths to weights. 15 ■ Fishing safely/safe boating . 17 ■ Food safety . 18 ■ Knots and rigs . 20 ■ Fish identification and measurement . 27 ■ Fish bag limits, size limits and closed seasons .