Chapter 3: Atmosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 2A Structure and Composition of the Earth Plate Tectonics
Engineering and Environmental Geology (EART 3012) – 2014 Lecture 2A Structure and composition of the Earth Plate tectonics Dr Tom Raimondo See Marshak pg. 42–53; 78–100 Figures taken from Earth: Portrait of a Planet, WW Norton & Co. Course outline and introduction Sweet weekly homework Every week, there are regular tasks that must be completed. There are clear expectations about the amount of time you should spend studying this course. Contact time per Non-contact time per week week Lectures 2 hours 1–2 hours pre- reading and revision Practicals 2 hours 1 hour pre-reading Weekly quizzes - 30 mins to 1 hour eModules - 30 mins to 1 hour Textbook online - 30 mins to 1 hour resources Total 4 hours 4–5 hours Lecture 2A Why do I need to know all this stuff? . Knowing the structure and composition of the Earth forms the basis for all geological concepts . We need to have a understanding of how the Earth behaves as a whole, and what its properties are, before we can consider more specific Earth systems and cycles . Plate tectonics is the fundamental geological theory for how the Earth works and how we can predict its behaviour . We need to understand this theory to be able to understand and interpret a range of geological phenomena (e.g. earthquakes, volcanoes, tsunamis, landslides, etc.) Lecture 2A Lecture outline Part 1: Structure and composition of the Earth . Layers of the Earth: crust, mantle and core . Lithosphere and asthenosphere Part 2: Plate tectonics . What is a tectonic plate? . Types of plate boundaries . Other plate features . -

Earth's Interior
11/7/2012 Please do the Audio Setup Wizard ! 11/7/12 Science Class Connect with Mrs. McFarland & Mr. Gluckin Earth’s Interior Ohio Academic Content Standards Today’s Class Agenda • Review Ground Rules for Earth and Space Sciences Classes The Universe • Earth’s Stats 9. Describe the interior structure of Earth and the Earth’s crust as • Composition of the Earth divided into tectonic plates riding on top of the slow moving currents of magma in the mantle. – Crust/Mantle/Core 11. Use models to analyze the size and shape of Earth, its surface and • Structures of the Earth its interior (e.g. globes, topographic maps and satellite images). – Lithosphere/Asthenosphere • Lithospheric Plates The Study Island lesson is due by 4pm on Thursday: SI 2e • State of matter and heat Student Centered Objectives – Convection Currents I will be able to describe the layers on the inside of the Earth. • Reminders I will be able use images to look at the Earth’s interior by composition. • Today’s Slides I will understand the different physical properties of the Earth’s layers. • Exit Ticket • Your Questions Ground rules Earth’s Stats • Please close all other apps & web pages. No Facebook, games, music, etc. • The Earth's mass is about 5.98 x 1024 kg. • No off‐topic chat • Be respectful of each other • Don’t share personal information • Earth is the densest planet in our Solar • I can see all chat … even “private chat” System (mass/volume). • Earth is made of several layers with different compositions and physical properties, like temperature, density, and the viscosity (“aka” ability to flow). -
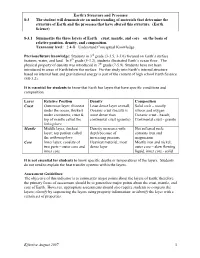
Earth's Structure and Processes 8-3 the Student Will Demonstrate An
Earth’s Structure and Processes 8-3 The student will demonstrate an understanding of materials that determine the structure of Earth and the processes that have altered this structure. (Earth Science) 8-3.1 Summarize the three layers of Earth – crust, mantle, and core – on the basis of relative position, density, and composition. Taxonomy level: 2.4-B Understand Conceptual Knowledge Previous/future knowledge: Students in 3rd grade (3-3.5, 3-3.6) focused on Earth’s surface features, water, and land. In 5th grade (5-3.2), students illustrated Earth’s ocean floor. The physical property of density was introduced in 7th grade (7-5.9). Students have not been introduced to areas of Earth below the surface. Further study into Earth’s internal structure based on internal heat and gravitational energy is part of the content of high school Earth Science (ES-3.2). It is essential for students to know that Earth has layers that have specific conditions and composition. Layer Relative Position Density Composition Crust Outermost layer; thinnest Least dense layer overall; Solid rock – mostly under the ocean, thickest Oceanic crust (basalt) is silicon and oxygen under continents; crust & more dense than Oceanic crust - basalt; top of mantle called the continental crust (granite) Continental crust - granite lithosphere Mantle Middle layer, thickest Density increases with Hot softened rock; layer; top portion called depth because of contains iron and the asthenosphere increasing pressure magnesium Core Inner layer; consists of Heaviest material; most Mostly iron and nickel; two parts – outer core and dense layer outer core – slow flowing inner core liquid, inner core - solid It is not essential for students to know specific depths or temperatures of the layers. -

Jet Streams Anomalies As Possible Short-Term Precursors Of
Research in Geophysics 2014; volume 4:4939 Jet streams anomalies as the existence of thermal fields, con nected with big linear structures and systems of crust Correspondence: Hong-Chun Wu, Institute of possible short-term precursors faults.2 Anomalous variations of latent heat Occupational Safety and Health, Safety of earthquakes with M 6.0 flow were observed for the 2003 M 7.6 Colima Department, Shijr City, Taipei, Taiwan. > (Mexico) earthquake.3 The atmospheric Tel. +886.2.22607600227 - Fax: +886.2.26607732. E-mail: [email protected] Hong-Chun Wu,1 Ivan N. Tikhonov2 effects of ionization are observed at the alti- 1Institute of Occupational Safety and tudes of top of the atmosphere (10-12 km). Key words: earthquake, epicenter, jet stream, pre- Health, Safety Department, Shijr City, Anomalous changes in outgoing longwave cursory anomaly. radiation were recorded at the height of 300 Taipei, Taiwan; 2Institute of Marine GPa before the 26 December 2004 M 9.0 Acknowledgments: the authors would like to Geology and Geophysics, FEB RAS; w express their most profound gratitude to the Indonesia earthquake.4 These anomalous ther- Yuzhno-Sakhalinsk, Russia National Center for Environmental Prediction of mal events occurred 4-20 days prior the earth- the USA and the National Earthquake quakes.5-8 Information Center on Earthquakes of the U.S. Local anomalous changes in the ionosphere Geological Survey for data on jet streams and 9-11 earthquakes on the Internet. Abstract over epicenters can precede earthquakes. Statistical analysis showed that these changes Dedications: the authors dedicate this work to occurred about 1-5 days before seismic the memory of Miss Cai Bi-Yu, who died in Satellite data of thermal images revealed events.12 To connect the listed above abnormal Taiwan during the 1999 earthquake with magni- the existence of thermal fields, connected with phenomena to preparation of earthquakes, tude M=7.3. -

Cold Plasma Diagnostics in the Jovian System
341 COLD PLASMA DIAGNOSTICS IN THE JOVIAN SYSTEM: BRIEF SCIENTIFIC CASE AND INSTRUMENTATION OVERVIEW J.-E. Wahlund(1), L. G. Blomberg(2), M. Morooka(1), M. André(1), A.I. Eriksson(1), J.A. Cumnock(2,3), G.T. Marklund(2), P.-A. Lindqvist(2) (1)Swedish Institute of Space Physics, SE-751 21 Uppsala, Sweden, E-mail: [email protected], [email protected], mats.andré@irfu.se, [email protected] (2)Alfvén Laboratory, Royal Institute of Technology, SE-100 44 Stockholm, Sweden, E-mail: [email protected], [email protected], [email protected], per- [email protected] (3)also at Center for Space Sciences, University of Texas at Dallas, U.S.A. ABSTRACT times for their atmospheres are of the order of a few The Jovian magnetosphere equatorial region is filled days at most. These atmospheres can therefore rightly with cold dense plasma that in a broad sense co-rotate with its magnetic field. The volcanic moon Io, which be termed exospheres, and their corresponding ionized expels sodium, sulphur and oxygen containing species, parts can be termed exo-ionospheres. dominates as a source for this cold plasma. The three Observations indicate that the exospheres of the three icy Galilean moons (Callisto, Ganymede, and Europa) icy Galilean moons (Europa, Ganymede and Callisto) also contribute with water group and oxygen ions. are oxygen rich [1, 2]. Several authors have also All the Galilean moons have thin atmospheres with modelled these exospheres [3, 4, 5], and the oxygen was residence times of a few days at most. -

Clay Model Earth
Teacher Instructions Clay Model Earth Overview: During this lesson students will learn about Earth’s layers by making a clay model. As a result of this activity, students develop an understanding of the structure of Earth’s layers, particularly as it relates to thickness and solid or molten state. Students keep completed Earth models in the classroom for future reference during Units 2 and 3. Note: You may wish to build a sample model Earth in the classroom, then have students build models as a homework assignment. Objectives: The student will learn: • to identify Earth’s layers, including the inner core, outer core, mantle, and crust; • the relative size and thickness of each Earth layer; • that Earth’s inner core is solid due to tremendous pressure; and • that Earth’s outer core is molten, and exists in a “liquid” state. Materials: • Clay in the colors and amounts described in the activity procedure (see Note, following page) • Marbles (25 mm) for each student • Sharp knife • Ruler • Teacher Information Sheet: “The Thickness and State of Earth’s Layers” • Transparency: “Earth’s Layers” • Transparency: “Clay Model Earth” • Student Worksheet: “Clay Model Earth” Answers to Student Worksheet: Crust 1. refer to diagram 2. outer core Mantle 3. mantle Molten 4. crust outer core 5. This is a critical thinking question; answers will vary. Solid inner core Ola Ka Honua: Volcanoes Alive 43 ©2001, 2007 UAF Geophysical Institute Teacher Instructions Clay Model Earth Activity Procedure: 1. Give a 25 mm marble and the following amounts of each color clay to each student: • One 4 oz. -

Moon–Magnetosphere Interactions: a Tutorial
Advances in Space Research 33 (2004) 2061–2077 www.elsevier.com/locate/asr Moon–magnetosphere interactions: a tutorial M.G. Kivelson a,b,* a University of California, Institute of Geophysics and Planetary Physics, UCLA, Los Angeles, CA 90095-1567, USA b Department of Earth and Space Sciences, UCLA, Los Angeles, CA 90095-1567, USA Received 8 May 2003; received in revised form 13 August 2003; accepted 18 August 2003 Abstract The interactions between the Galilean satellites and the plasma of the Jovian magnetosphere, acting on spatial scales from that of ion gyroradii to that of magnetohydrodynamics (MHD), change the plasma momentum, temperature, and phase space distribution functions and generate strong electrical currents. In the immediate vicinity of the moons, these currents are often highly structured, possibly because of varying ionospheric conductivity and possibly because of non-uniform pickup rates. Ion pickup changes the velocity space distribution of energetic particles, f (v), where v is velocity. Distributions become more anisotropic and can become unstable to wave generation. That there would be interesting plasma responses near Io was fully anticipated, but one of the surprises of Galileo’s mission was the range of effects observed at all of the Galilean satellites. Electron beams and an assortment of MHD and plasma waves develop in the regions around the moons, although each interaction region is different. Coupling of the plasma near the moons to the Jovian ionosphere creates auroral footprints and, in the case of Io, produces a leading trail in the ionosphere that extends almost half way around Jupiter. The energy source driving the auroral signatures is not fully understood but must require field aligned electric fields that accelerate elections at the feet of the flux tubes of Io and Europa, bodies that interact directly with the incident plasma, and at the foot of the flux tube of Ganymede, a body that is shielded from direct interaction with the background plasma by its magnetospheric cavity. -

The Magnetosphere-Ionosphere Observatory (MIO)
1 The Magnetosphere-Ionosphere Observatory (MIO) …a mission concept to answer the question “What Drives Auroral Arcs” ♥ Get inside the aurora in the magnetosphere ♥ Know you’re inside the aurora ♥ Measure critical gradients writeup by: Joe Borovsky Los Alamos National Laboratory [email protected] (505)667-8368 updated April 4, 2002 Abstract: The MIO mission concept involves a tight swarm of satellites in geosynchronous orbit that are magnetically connected to a ground-based observatory, with a satellite-based electron beam establishing the precise connection to the ionosphere. The aspect of this mission that enables it to solve the outstanding auroral problem is “being in the right place at the right time – and knowing it”. Each of the many auroral-arc-generator mechanisms that have been hypothesized has a characteristic gradient in the magnetosphere as its fingerprint. The MIO mission is focused on (1) getting inside the auroral generator in the magnetosphere, (2) knowing you are inside, and (3) measuring critical gradients inside the generator. The decisive gradient measurements are performed in the magnetosphere with satellite separations of 100’s of km. The magnetic footpoint of the swarm is marked in the ionosphere with an electron gun firing into the loss cone from one satellite. The beamspot is detected from the ground optically and/or by HF radar, and ground-based auroral imagers and radar provide the auroral context of the satellite swarm. With the satellites in geosynchronous orbit, a single ground observatory can spot the beam image and monitor the aurora, with full-time conjunctions between the satellites and the aurora. -

High School - Earth and Space Science
High School - Earth and Space Science Grade Big Idea Essential Questions Concepts Competencies Vocabulary 2002 SAS Assessment Standards Standards Anchor Eligible Content The universe is What is the universe and The Milky Way Galaxy consists of Use models to describe the Clusters 3.4.10.D 3.3.10.B1 composed of a variety what is Earth’s place in more than two hundred billion sun’s place in space in relation Galaxy of different objects that it? stars, the sun being one of them, to the Milky Way Galaxy and the Model are organized into and is one of hundreds of billions distribution of galaxy clusters in Star 9-12 systems each of which of galaxies in the known universe. the universe. Universe develops according to accepted physical processes and laws. The universe is What is the universe and Models of the formation and Compare time periods in history, Geocentric 3.1.12.E 3.4.10.B composed of a variety what is Earth’s place in structure of the universe have the technology available at that Heliocentric 3.4.10.D3 of different objects that it? changed over time as technologies time and the resulting model of Model are organized into have become more advanced and the organization of our solar Planet 9-12 systems each of which the accuracy of our data has system. (e.g. – Early Greeks Theory develops according to increased. used purely observational data accepted physical resulting in a geocentric model). processes and laws. The universe is What is the universe and The Milky Way Galaxy consists of Use data about the expansion, Clusters 3.4.10.D 3.3.10.B1 composed of a variety what is Earth’s place in more than two hundred billion scale and age of the universe to Galaxy 3.3.12.B2 of different objects that it? stars, the sun being one of them, explain the Big Bang theory as a Light year are organized into and is one of hundreds of billions model for the origin of the Model 9-12 systems each of which of galaxies in the known universe. -

Framework and NGSS
POPULATIONS AND ECOSYSTEMS – Framework and NGSS INTRODUCTION TO Contents PERFORMANCE EXPECTATIONS Introduction to Performance “The NGSS are standards or goals, that reflect what a student Expectations .......................... 35 should know and be able to do; they do not dictate the manner or methods by which the standards are taught. Curriculum FOSS Conceptual and assessment must be developed in a way that builds students’ Framework ............................ 48 knowledge and ability toward the PEs [performance expectations]” Background for the (Next Generation Science Standards, 2013, page xiv). Conceptual Framework .......... 50 This chapter shows how the NGSS Performance Expectations are Connections to NGSS by bundled in the Populations and Ecosystems Course to provide Investigation .......................... 56 a coherent set of instructional materials for teaching and learning. This chapter also provides details about how this FOSS course Recommended FOSS Next fits into the matrix of the FOSS Program (page 49). Each FOSS Generation K–8 Scope and module K–5 and middle school course 6–8 has a functional role Sequence ............................... 74 in the FOSS conceptual frameworks that were developed based on a decade of research on science education and the influence of A Framework for K–12 Science Education (2012) and Next Generation Science Standards (NGSS, 2013). The FOSS curriculum provides a coherent vision of science teaching and learning in the three ways described by the The NGSS Performance Expectations NRC Framework. First, FOSS is designed around learning as bundled in this course include a developmental progression, providing experiences that allow students to continually build on their initial notions and develop Life Sciences more complex science and engineering knowledge. -

Radio Sounding of the Venusian Atmosphere and Ionosphere with Envision
EPSC Abstracts Vol. 13, EPSC-DPS2019-609-1, 2019 EPSC-DPS Joint Meeting 2019 c Author(s) 2019. CC Attribution 4.0 license. Radio Sounding of the Venusian Atmosphere and Ionosphere with EnVision Silvia Tellmann (1), Yohai Kaspi (2), Sébastien Lebonnois (3) , Franck Lefèvre (4), Janusz Oschlisniok (1), Paul Withers (5), Caroline Dumoulin (6), and Pascal Rosenblatt (6,7) (1) Rheinisches Institut für Umweltforschung, Abteilung Planetenforschung, Universität zu Köln, Cologne, Germany, (2) Department of Earth and Planetary Sciences, Weizmann Institute of Science, Rehovot, Israel, (3) LMD/IPSL, Sorbonne Université, CNRS, Paris, France, (4) LATMOS, CNRS/Sorbonne Université, Paris,(5) Astronomy Department, Boston University, Boston, MA, USA, (6) Laboratoire de Planétologie et Géodynamique, Université de Nantes, France, (7) Geoazur, Nice Sophia-Antipolis University, France, ([email protected]) Abstract temperature and pressure profiles in the mesosphere and upper troposphere of Venus (~ 40 - 90 km). The EnVision is a one of the final candidates for the M5 first radio occultation experiment at Venus was call of the Cosmic Vision program from ESA. It is conducted during the Mariner 5 flyby in 1967 [2], dedicated to unravel some of the numerous open followed by Mariner 10 [3], several Venera missions questions about Venus' past, current state and future. [4], Magellan [5] and the Pioneer Venus Orbiter [6], The Radio Science Experiment on EnVision will and Akatsuki [7]. The most extensive radio perform extensive studies of the gravitational field occultation study of the Venus atmosphere so far was but also Radio Occultations to sense the Venus carried out by the VeRa experiment on Venus atmosphere and ionosphere at a high vertical Express [8,9]. -

An Introduction to Geology Focus on Concepts Each Statement Represents the Primary Learning Objective for the Corresponding Major Heading Within the Chapter
1An Introduction to Geology FOCUS ON CONCEPTS Each statement represents the primary learning objective for the corresponding major heading within the chapter. After you complete the chapter, you should be able to: 1.1 Distinguish between physical and historical geology and describe the connections between people and geology. 1.2 Summarize early and modern views on how change occurs on Earth and relate them to the prevailing ideas about the age of Earth. 1.3 Discuss the nature of scientific inquiry, including the construction of hypotheses and the development of theories. 1.4 List and describe Earth’s four major spheres. Define system and explain why Earth is considered to be a system. 1.5 Outline the stages in the formation of our solar system. 1.6 Describe Earth’s internal structure. 1.7 Sketch, label, and explain the rock cycle. 1.8 List and describe the major features of the continents and ocean basins. All four of Earth’s spheres are represented in this image in the Canadian Rockies of British Columbia. (Photo by CCOphotostock_KMN) 2 M01_TARB6622_13_SE_C01.indd 2 11/11/16 12:55 PM 2 M01_TARB6622_13_SE_C01.indd 3 11/11/16 12:55 PM THE SPECTACULAR ERUPTION OF A VOLCANO, the terror brought by an earth- quake, the magnificent scenery of a mountain range, and the destruction created by a landslide or flood are all subjects for a geologist. The study of geology deals with many fascinating and practical questions about our physical environment. What forces produce mountains? When will the next major earthquake occur in California? What are ice ages like, and will there be another? How were ore deposits formed? Where should we search for water? Will plentiful oil be found if a well is drilled in a particular location? Geologists seek to answer these and many other questions about Earth, its history, and its resources.