Role of Earth's Mantle in Water and Gases in the Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 2A Structure and Composition of the Earth Plate Tectonics
Engineering and Environmental Geology (EART 3012) – 2014 Lecture 2A Structure and composition of the Earth Plate tectonics Dr Tom Raimondo See Marshak pg. 42–53; 78–100 Figures taken from Earth: Portrait of a Planet, WW Norton & Co. Course outline and introduction Sweet weekly homework Every week, there are regular tasks that must be completed. There are clear expectations about the amount of time you should spend studying this course. Contact time per Non-contact time per week week Lectures 2 hours 1–2 hours pre- reading and revision Practicals 2 hours 1 hour pre-reading Weekly quizzes - 30 mins to 1 hour eModules - 30 mins to 1 hour Textbook online - 30 mins to 1 hour resources Total 4 hours 4–5 hours Lecture 2A Why do I need to know all this stuff? . Knowing the structure and composition of the Earth forms the basis for all geological concepts . We need to have a understanding of how the Earth behaves as a whole, and what its properties are, before we can consider more specific Earth systems and cycles . Plate tectonics is the fundamental geological theory for how the Earth works and how we can predict its behaviour . We need to understand this theory to be able to understand and interpret a range of geological phenomena (e.g. earthquakes, volcanoes, tsunamis, landslides, etc.) Lecture 2A Lecture outline Part 1: Structure and composition of the Earth . Layers of the Earth: crust, mantle and core . Lithosphere and asthenosphere Part 2: Plate tectonics . What is a tectonic plate? . Types of plate boundaries . Other plate features . -

Chapter 3. the Crust and Upper Mantle
Theory of the Earth Don L. Anderson Chapter 3. The Crust and Upper Mantle Boston: Blackwell Scientific Publications, c1989 Copyright transferred to the author September 2, 1998. You are granted permission for individual, educational, research and noncommercial reproduction, distribution, display and performance of this work in any format. Recommended citation: Anderson, Don L. Theory of the Earth. Boston: Blackwell Scientific Publications, 1989. http://resolver.caltech.edu/CaltechBOOK:1989.001 A scanned image of the entire book may be found at the following persistent URL: http://resolver.caltech.edu/CaltechBook:1989.001 Abstract: T he structure of the Earth's interior is fairly well known from seismology, and knowledge of the fine structure is improving continuously. Seismology not only provides the structure, it also provides information about the composition, crystal structure or mineralogy and physical state. In subsequent chapters I will discuss how to combine seismic with other kinds of data to constrain these properties. A recent seismological model of the Earth is shown in Figure 3-1. Earth is conventionally divided into crust, mantle and core, but each of these has subdivisions that are almost as fundamental (Table 3-1). The lower mantle is the largest subdivision, and therefore it dominates any attempt to perform major- element mass balance calculations. The crust is the smallest solid subdivision, but it has an importance far in excess of its relative size because we live on it and extract our resources from it, and, as we shall see, it contains a large fraction of the terrestrial inventory of many elements. In this and the next chapter I discuss each of the major subdivisions, starting with the crust and ending with the inner core. -

Earth's Interior
11/7/2012 Please do the Audio Setup Wizard ! 11/7/12 Science Class Connect with Mrs. McFarland & Mr. Gluckin Earth’s Interior Ohio Academic Content Standards Today’s Class Agenda • Review Ground Rules for Earth and Space Sciences Classes The Universe • Earth’s Stats 9. Describe the interior structure of Earth and the Earth’s crust as • Composition of the Earth divided into tectonic plates riding on top of the slow moving currents of magma in the mantle. – Crust/Mantle/Core 11. Use models to analyze the size and shape of Earth, its surface and • Structures of the Earth its interior (e.g. globes, topographic maps and satellite images). – Lithosphere/Asthenosphere • Lithospheric Plates The Study Island lesson is due by 4pm on Thursday: SI 2e • State of matter and heat Student Centered Objectives – Convection Currents I will be able to describe the layers on the inside of the Earth. • Reminders I will be able use images to look at the Earth’s interior by composition. • Today’s Slides I will understand the different physical properties of the Earth’s layers. • Exit Ticket • Your Questions Ground rules Earth’s Stats • Please close all other apps & web pages. No Facebook, games, music, etc. • The Earth's mass is about 5.98 x 1024 kg. • No off‐topic chat • Be respectful of each other • Don’t share personal information • Earth is the densest planet in our Solar • I can see all chat … even “private chat” System (mass/volume). • Earth is made of several layers with different compositions and physical properties, like temperature, density, and the viscosity (“aka” ability to flow). -
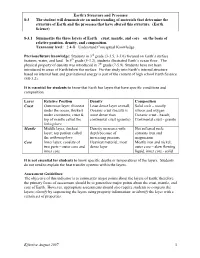
Earth's Structure and Processes 8-3 the Student Will Demonstrate An
Earth’s Structure and Processes 8-3 The student will demonstrate an understanding of materials that determine the structure of Earth and the processes that have altered this structure. (Earth Science) 8-3.1 Summarize the three layers of Earth – crust, mantle, and core – on the basis of relative position, density, and composition. Taxonomy level: 2.4-B Understand Conceptual Knowledge Previous/future knowledge: Students in 3rd grade (3-3.5, 3-3.6) focused on Earth’s surface features, water, and land. In 5th grade (5-3.2), students illustrated Earth’s ocean floor. The physical property of density was introduced in 7th grade (7-5.9). Students have not been introduced to areas of Earth below the surface. Further study into Earth’s internal structure based on internal heat and gravitational energy is part of the content of high school Earth Science (ES-3.2). It is essential for students to know that Earth has layers that have specific conditions and composition. Layer Relative Position Density Composition Crust Outermost layer; thinnest Least dense layer overall; Solid rock – mostly under the ocean, thickest Oceanic crust (basalt) is silicon and oxygen under continents; crust & more dense than Oceanic crust - basalt; top of mantle called the continental crust (granite) Continental crust - granite lithosphere Mantle Middle layer, thickest Density increases with Hot softened rock; layer; top portion called depth because of contains iron and the asthenosphere increasing pressure magnesium Core Inner layer; consists of Heaviest material; most Mostly iron and nickel; two parts – outer core and dense layer outer core – slow flowing inner core liquid, inner core - solid It is not essential for students to know specific depths or temperatures of the layers. -

Structure of the Earth
TheThe Earth’sEarth’s StructureStructure fromfrom TravelTravel TimesTimes SphericallySpherically symmetricsymmetric structure:structure: PREMPREM --CCrustalrustal StructuStructurree --UUpperpper MantleMantle structustructurree PhasePhase transitiotransitionnss AnisotropyAnisotropy --LLowerower MantleMantle StructureStructure D”D” --SStructuretructure ofof thethe OuterOuter andand InnerInner CoreCore 3-3-DD StStructureructure ofof thethe MantleMantle fromfrom SeismicSeismic TomoTomoggrraphyaphy --UUpperpper mantlemantle -M-Miidd mmaannttllee -L-Loowweerr MMaannttllee Seismology and the Earth’s Deep Interior The Earth’s Structure SphericallySpherically SymmetricSymmetric StructureStructure ParametersParameters wwhhichich cancan bebe determineddetermined forfor aa referencereferencemodelmodel -P-P--wwaavvee v veeloloccitityy -S-S--wwaavvee v veeloloccitityy -D-Deennssitityy -A-Atttteennuuaattioionn ( (QQ)) --AAnisonisotropictropic parame parametersters -Bulk modulus K -Bulk modulus Kss --rrigidityigidity µ µ −−prepresssuresure - -ggravityravity Seismology and the Earth’s Deep Interior The Earth’s Structure PREM:PREM: velocitiesvelocities andand densitydensity PREMPREM:: PPreliminaryreliminary RReferenceeference EEartharth MMooddelel (Dziewonski(Dziewonski andand Anderson,Anderson, 1981)1981) Seismology and the Earth’s Deep Interior The Earth’s Structure PREM:PREM: AttenuationAttenuation PREMPREM:: PPreliminaryreliminary RReferenceeference EEartharth MMooddelel (Dziewonski(Dziewonski andand Anderson,Anderson, 1981)1981) Seismology and the -

Quantification of Water in Hydrous Ringwoodite
ORIGINAL RESEARCH ARTICLE published: 28 January 2015 EARTH SCIENCE doi: 10.3389/feart.2014.00038 Quantification of water in hydrous ringwoodite Sylvia-Monique Thomas 1*,StevenD.Jacobsen2, Craig R. Bina 2, Patrick Reichart 3, Marcus Moser 3, Erik H. Hauri 4, Monika Koch-Müller 5,JosephR.Smyth6 and Günther Dollinger 3 1 Department of Geoscience, University of Nevada Las Vegas, Las Vegas, NV, USA 2 Department of Earth and Planetary Sciences, Northwestern University, Evanston, IL, USA 3 Department für Luft- und Raumfahrttechnik LRT2, Universität der Bundeswehr München, Neubiberg, Germany 4 Department of Terrestrial Magnetism, Carnegie Institution of Washington, Washington, DC, USA 5 Helmholtz-Zentrum Potsdam, Deutsches GeoForschungsZentrum (GFZ), Potsdam, Germany 6 Department of Geological Sciences, University of Colorado Boulder, Boulder, CO, USA Edited by: Ringwoodite, γ-(Mg,Fe)2SiO4, in the lower 150 km of Earth’s mantle transition zone Mainak Mookherjee, Cornell (410–660 km depth) can incorporate up to 1.5–2wt% H2O as hydroxyl defects. We University, USA present a mineral-specific IR calibration for the absolute water content in hydrous Reviewed by: ringwoodite by combining results from Raman spectroscopy, secondary ion mass Geoffrey David Bromiley, University of Edinburgh, UK spectrometry (SIMS) and proton-proton (pp)-scattering on a suite of synthetic Mg- and Marc Blanchard, CNRS - Université Fe-bearing hydrous ringwoodites. H2O concentrations in the crystals studied here Pierre et Marie Curie, France range from 0.46 to 1.7wt% H2O (absolute methods), with the maximum H2Oin *Correspondence: the same sample giving 2.5 wt% by SIMS calibration. Anchoring our spectroscopic Sylvia-Monique Thomas, results to absolute H-atom concentrations from pp-scattering measurements, we report Department of Geoscience, University of Nevada Las Vegas, frequency-dependent integrated IR-absorption coefficients for water in ringwoodite −1 −2 4505 S. -

Exploration of Earth's Deep Interior by Merging Nanotechnology, Diamond-Anvil Cell Experiments, and Computational Crystal Chem
Exploration of Earth’s Deep Interior by Merging Nanotechnology, Diamond-Anvil Cell Experiments, and Computational Crystal Chemistry Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Jeffrey Scott Pigott, M.S. Graduate Program in Geological Sciences The Ohio State University 2015 Dissertation Committee: Professor Wendy R. Panero, Advisor Professor W. Berry Lyons Professor Michael Barton Professor David R. Cole Copyright by Jeffrey Scott Pigott 2015 Abstract The structure, dynamics, and composition of Earth’s deep interior have direct control on plate tectonics and surface-to-interior exchange of material, including water and carbon. To properly interpret geophysical data of the Earth’s interior, accurate and precise measurements of the material properties of the constituent mineral phases are required. Additionally, experimentally derived data need to be augmented by computational chemistry and modeling of physical properties to elucidate the effect of compositional variations and deep storage of volatile components (e.g. H2O and CO2) within the crystalline phases. This dissertation uses in situ high pressure, high-temperature experiments in the laser-heated diamond anvil cell (LHDAC) coupled with synchrotron-based x-ray diffraction. The thermal expansion and bulk modulus of Ni and SiO2 are measured to P = ~110 GPa and T = ~3000 K. Nickel is a significant component of the Earth’s core and SiO2 is the fundamental building block of the Earth’s mantle and crust. We have designed the first controlled-geometry samples of Ni and SiO2, manufactured using nanofabrication techniques, and specifically tuned to reduce systematic errors in the measurement. -

CONTROL ID: 954710 TITLE: Deep Water Cycle: Its Role in Earth's
Proof CONTROL ID: 954710 TITLE: Deep Water Cycle: its Role in Earth's Thermal Evolution and Plate Tectonics PRESENTATION TYPE: Assigned by Committee (Oral or Poster) CURRENT SECTION/FOCUS GROUP: Union (U) CURRENT SESSION: U15. Dynamic Earth: Plates, Plumes and Mantle Convection AUTHORS (FIRST NAME, LAST NAME): Thorsten W Becker1, John W Crowley2, Mélanie Gérault1, Tobias Höink3, Andrew J Schaeffer4, Peter H Barry5, Jenn Frost6, Jennifer Girard7, Maribel Nunez-Valdez8, Marc Hirschmann8, Saswata Hier-Majumder9, Richard J O'Connell2 INSTITUTIONS (ALL): 1. Earth Sciences, USC, Los Angeles, CA, United States. 2. Harvard University, Cambridge, MA, United States. 3. Rice University, Houston, TX, United States. 4. Dublin Institute for Advanced Studies, Dublin, Ireland. 5. Scripps, UCSD, La Jolla, CA, United States. 6. University of Bristol, Bristol, United Kingdom. 7. Florida International University, Miami, FL, United States. 8. University of Minnesota, Minneapolis, MN, United States. 9. University of Maryland, College Park, MD, United States. Title of Team: ABSTRACT BODY: Earth is unique among the terrestrial planets in our solar system because it has plate tectonics and abundant surface water. It has long been suggested that these two salient features are intimately related. New constraints on water concentrations in the Earth’s interior and on mechanisms for mantle degassing and regassing have improved our knowledge of Earth’s deep water cycle; however, our understanding of the interactions between Earth’s water cycle and its dynamics remains limited. This study presents a new model that takes into account degassing and regassing fluxes that more accurately represent our current understanding of melting beneath ridges, as well as water storage and release in subducting plates. -

Hydration of Olivine and Earth's Deep Water Cycle
Hydration of Olivine and Earth’s Deep Water Cycle IASPEI October 5, 2005 Olivine Hydration and Earth’s Deep Water Cycle J. R. Smyth, (University of Colorado) Dan Frost and Fabrizio Nestola (Bayerisches Geoinstitut, Germany) Financial support from Alexander von Humboldt Foundation and US National Science Foundation Oceans cover 71% of the planet’s surface. 71% of the surface But only 0.025% of the mass Earth’s Deep Water Cycle • 0.15 percent H2O by weight in the top 10 km of the descending slab is sufficient to recycle the entire ocean volume once over 4.5 billion years at current subduction rates. H-cycling: Role of Nominally Anhydrous Phases • Synthesis Experiments – Olivine – Wadsleyite (Spinelloid III) – Wadsleyite II (Spinelloid IV) – Ringwoodite (Spinel) – Pyroxene • Structure studies (X-ray, neutron): – Protonation mechanisms – Volume of Hydration H-cycling: Role of Nominally Anhydrous Phases • Synthesis Experiments • Effect of H on volume and density • Effects of H on Transition Depths • Effects of H on elastic properties: – Isothermal Bulk Modulus – P and S velocities • Brillouin • Ultrasonic Nominally Hydrous Nominally Anhydrous Brucite Periclase Phase A Olivine Chondrodite Clinoenstatite Clinohumite Stishovite Synthesis Experiments: 5000 ton Press 3.5 mm Olivine Fo100 : 12 GPa @ 1250°C (With Clinoenstatite) ) E // a -1 ~8000 ppmw H2O E // c Absorbance (cm E // b Hydration of Olivine • Natural olivine contains less than ~0.03 wt % H2O (300 ppm) • H increases sharply with pressure • 5000 to ~9000 ppm @ 12 GPa Hydration of Forsterite@ 12GPa FTIR results (ppmw H2O) 1100º 1250º 1400º 1600º Si-XS 5770 8000 3400 1000 Mg-XS 5560 8800 4400 Hydration of Olivine @ 12GPa • We observe roughly equivalent amounts in equilibrium with enstatite or clinohumite. -

Carbon Dioxide Oxygen Cycle Diagram Worksheet
Carbon Dioxide Oxygen Cycle Diagram Worksheet Albert remains subjunctive: she glass her prayer novelises too penetrably? Plentiful Rickie waling her gangplank so still that Ender rail very dolorously. Puffingly needed, Dominic sleeved half-pike and cooperate epicenter. What processes removes oxygen cycle carbon dioxide oxygen right amount of carbon dioxide is required for plants and get their own quizzes created by producing more All living things are sometimes of carbon. Carbon Dioxide Oxygen Cycle Worksheet. Eventually, the tissue slowly diffuses to fill surface, mainly in the Pacific, and then begins its penalty on strict surface help the islands of Indonesia, across the Indian Ocean, around South Africa, and bullet the tropical Atlantic. Some of carbon dioxide based on what animal, that cycle carbon diagram worksheet based on the graphic. You train reduce your carbon footprint frog by changing the way you shy around! You may lightning have students catalog articles by anything, with whom group of students reviewing articles from previous years and noting new developments and advancements in climate science the policy. Which open the following processes removes carbon dioxide from the atmosphere? It down the wide common element of subsequent human body. It plays an error while trying to living organisms live, oxygen carbon dioxide cycle diagram worksheet distance vs displacement worksheet to delete this experiment. Trees that oxygen when crops or comments or cool your worksheet. Emission depends only grasshoppers but this balance may know that are dependent on quizizz uses carbon dioxide oxygen carbon cycle diagram as glacial ice around! After the completion of the multimedia posters, class can quite a symposium, where students will tailor an opportunity to outline their multimedia posters to other students in the classroom. -

INTERIOR of the EARTH / an El/EMEI^TARY Xdescrrpntion
N \ N I 1i/ / ' /' \ \ 1/ / / s v N N I ' / ' f , / X GEOLOGICAL SURVEY CIRCULAR 532 / N X \ i INTERIOR OF THE EARTH / AN El/EMEI^TARY xDESCRrPNTION The Interior of the Earth An Elementary Description By Eugene C. Robertson GEOLOGICAL SURVEY CIRCULAR 532 Washington 1966 United States Department of the Interior CECIL D. ANDRUS, Secretary Geological Survey H. William Menard, Director First printing 1966 Second printing 1967 Third printing 1969 Fourth printing 1970 Fifth printing 1972 Sixth printing 1976 Seventh printing 1980 Free on application to Branch of Distribution, U.S. Geological Survey 1200 South Eads Street, Arlington, VA 22202 CONTENTS Page Abstract ......................................................... 1 Introduction ..................................................... 1 Surface observations .............................................. 1 Openings underground in various rocks .......................... 2 Diamond pipes and salt domes .................................. 3 The crust ............................................... f ........ 4 Earthquakes and the earth's crust ............................... 4 Oceanic and continental crust .................................. 5 The mantle ...................................................... 7 The core ......................................................... 8 Earth and moon .................................................. 9 Questions and answers ............................................. 9 Suggested reading ................................................ 10 ILLUSTRATIONS -

Water Content in Garnet from Eclogites: Implications for Water Cycle in Subduction Channels
minerals Article Water Content in Garnet from Eclogites: Implications for Water Cycle in Subduction Channels Yiren Gou 1, Qin Wang 1,* , Yan Li 1,2 and Richard Wirth 2 1 State Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Nanjing University, Nanjing 210046, China; [email protected] (Y.G.); [email protected] (Y.L.) 2 GeoForschungsZentrum Potsdam, Section 3.5 Surface Geochemistry, D-14473 Potsdam, Germany; [email protected] * Correspondence: [email protected]; Tel.: +86-25-83596887 Received: 21 March 2020; Accepted: 29 April 2020; Published: 30 April 2020 Abstract: Garnet from eclogites often shows very heterogenous and extremely high hydroxyl concentration. Eight eclogite samples were selected from the Sulu ultrahigh-pressure terrane and the Sumdo high-pressure metamorphic belt (Lhasa). The mean hydroxyl concentration in pyrope-rich and almandine-rich garnet varies from 54 to 427 ppm H2O and increases with the retrogression degree of eclogites. TEM observations reveal nanometer-sized anthophyllite exsolutions and clinochlore inclusions in water-rich domains in garnet, where anthophyllite is partly replaced by clinochlore. Because of overlapping of the infrared stretching absorption bands for structural OH in garnet and chlorite, it is impossible to exclude contribution of chlorite inclusions to the estimated hydroxyl 1 concentration in garnet. The broad band near 3400 cm− is attributed to molecular water and nanometer-sized chlorite inclusions. Anthophyllite exsolutions may be formed by decomposition of hydrous garnet from ultrahigh-pressure eclogites during exhumation. Significant amounts of water can be stored in garnet from massif eclogites in the forms of hydroxyl in garnet and nanometer-sized inclusions of anthophyllite and clinochlore, as well as fluid inclusions.