Assessment of a 72-Hour Repeated Exposure to Swedish Snus Extract
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Oil Outlook 2040
Organization of the Petroleum Exporting Countries 2019 World Oil Outlook 2040 2019 World Oil Outlook 2040 Organization of the Petroleum Exporting Countries Digital access to the WOO: an interactive user experience 24/7 OPEC’s World Oil Outlook (WOO) is part of the Organization’s commitment to market stability. The publication is a means to highlight and further the understanding of the many possible future challenges and opportunities for the oil industry. It is also a channel to encourage dialogue, cooperation and transparency between OPEC and other stakeholders within the industry. As part of OPEC’s ongoing efforts to improve user experience of the WOO and provide data transparency, two digital interfaces are available: the OPEC WOO App and the interactive version of the WOO. The OPEC WOO App provides increased access to the publication’s vital analysis and energy-related data. It is ideal for energy professionals, oil industry stakeholders, policymakers, market analysts, academics and the media. The App’s search engine enables users to easily find information, and its bookmarking function allows them to store and review their favourite articles. Its versatility also allows users to compare graphs and tables interactively, thereby maximizing information extraction and empowering users to undertake their own analysis. The interactive version of the WOO also provides the possibility to download specific data and information, thereby enhancing user experience. Download Access the OPEC WOO App interactive version Available for Android and iOS OPEC is a permanent, intergovernmental organization, established in Baghdad, Iraq, on 10–14 September 1960. The Organization comprises 14 Members: Algeria, Angola, Republic of the Congo, Ecuador, Equatorial Guinea, Gabon, the Islamic Republic of Iran, Iraq, Kuwait, Libya, Nigeria, Saudi Arabia, the United Arab Emirates and Venezuela. -

The Woo the Home Builder an Independent Newspaper Published in the Interest of Woodbridge Township
WOOBBRIDGE TOWNSHIP WOOnBK IDG R TOWNSHt V IDEAL WELCOMES INDUSTRIAL SITES THE WOO THE HOME BUILDER AN INDEPENDENT NEWSPAPER PUBLISHED IN THE INTEREST OF WOODBRIDGE TOWNSHIP SEVENTEENTH YEAR Woodbridge, N. X, Friday Afternoon, April 22, 1927 PRICE THREE CENTS—$1.50 PEE YEAR WOODBRIDGE Daylight Time Starts .c. I Miss Duff First Woman Queer Weather, not MAN'S FACE Monday at 2 A. M. \ to Open Law Office Here Dae to Tornado In TOWNSHIP IS Daylight Saving Time, the TO STAGE BIG the Middle West CUT IN HALF bane of farmers; but the boon of factory and office' workers People are wondering what will start at 2 A. M. next Mon- SHOW NEXT thp weather will be. like for the WHEN AUTO day morning. Turn, your clock next month or so, after experi- J] ahead one hour before going to encing the sudden heat wave on .si bed Sunday night, and you TUESDAY EVE Wednesday, followed by a ter- HITS POLE •won't be late on the job the" rific showers and electrical dis- next morning. If you forget turbance last night. In Middlesex County, ( you'll hare to make a lame ex- Annual Laiigh-Fest Expected! In an interview with fan V. S. Matthew J. Lund of Keasbey, cuss to the boss. W-:ulu>r Uuivau at the White- Board's Report . Shows, j The periodic advancing of the to Draw Record Crowd to. Hail Huildin;:, ls>w York Cky Driver of Car, Unhurt. Figures Reveal Startling | clock gives all workers the bene- St. James Auditorium. \ this morning, the Leael-r-r was fit or an extra hour of sunlight tolu that tli? wr-athw would I Drove Car in Which Man Possibilities of Expansion. -

Episode: #36 Puppy Selection: How I Do It and Why I Didn’T This! Time
Episode: #36 Puppy Selection: How I Do It and Why I Didn’t This! Time Speaker Key SG Susan Garrett Transcript 00:00:10 SG Hey everybody, welcome to Shaped by Dog. I am Susan Garrett, and today is the big day. On today's podcast I am going to answer the number one question I've been asked all over social media in the last month and a half. What puppy am I picking? I'm going to share with you how I go about picking a puppy, either for myself, or if a student asks me to pick a puppy for them, this is how I go about doing it, which I rarely do anymore, but I'm going to start with a review. I love reading your reviews. So please, please, please wherever you are listening to this podcast, leave me a review. And if you are listening to this podcast, I strongly encourage you to go on over to YouTube, youtube.com/dogsthat and watch this episode. This episode in particular, lots of graphics to go with this one. 00:01:09 Alright. This review. It comes from a listener in Great Britain with the handle of Agilitysequin, and the title of this review is ‘More gems’. “I've been following Susan for years and these podcasts are adding another layer to my dog training and my life in general. They make me a better person, a better dog owner, a better parent and look at life in a different way. I'll give you an example.” I love this story by the way. -

Midyear Report Canada 2020
NIELSEN MUSIC / MRC DATA MIDYEAR REPORT CANADA 2020 1 Introduction HAT A DIFFERENCE A FEW MONTHS MAKE. IT’S HARD TO BELIEVE THAT IT WASN’T even six months ago that Shakira and Jennifer Lopez performed for a tightly packed crowd of more than 60,000 people in Miami at the Super Bowl, while Billie Eilish and her brother Finneas picked up five Grammys at what turned out to be 2020’s last major music business gathering since COVID-19 halted live events. WBy Friday, March 13, the NBA and NHL had suspended play, the NCAA had canceled its spring tournaments, and theaters and live music venues had closed. As “safer at home” orders spread throughout the country, our homes became our offices, our schools, our daycares and our social hubs. Quickly and dramatically, life had changed. Our routines were disrupted, and we struggled to find balance. Still, the music industry was experiencing a strong start to the year. Audio streaming was growing through early March, up 20.9% over the same period in 2019. Total music consumption was up 10.8% in the first 10 weeks of the year. As working from home became a reality for many, some of the key music listening hours, such as during commutes, were disrupted. But one thing that has remained consistent as the pandemic has unfolded is entertainment’s place in helping consumers escape, relax and feel energized. In fact, in our recent consumer research studies, 73% of people said they would go crazy without entertainment during this time. Then, just as many communities began to slowly reopen, the country was shaken by the senseless May 25 killing of George Floyd by Minneapolis police. -

The Woo Lyrics – Pop Smoke {50 Cent
The Woo Lyrics – Pop Smoke {50 Cent & Roddy Ricch} The woo lyrics – The woo Lyrics – Description:- The woo Lyrics Pop Smoke ft. Lil Baby & DaBaby are Provided in this article. This is a new song which is sang by famous singer Pop Smoke. This song is from Shoot for the Stars, Aim for the Moon album. This Song will release on 3 July 2020. The woo lyrics [Intro: Pop Smoke] She want a Woo nigga [Chorus: Pop Smoke] She wanna fuck with the Woo She wanna fuck with the Woo She wanna fuck with the Woo She wanna fuck with the Woo Yeah, yeah, yeah, yeah [Verse 1: Roddy Ricch] I told her, “Ooh-woo-woo” Shawty wanna fuck me inside of the coupe I can take you out everywhere, fuck a jet fare Versace hotel with Versace robe, woah, woah Like you when you let down your hair with no comb And I stay to myself ’cause I never liked these hoes (Yeah) And she only liked the guap, red light these hoes Why would I waste my time on a shorty that don’t got me on the front of her mind? Especially when you get designer and I wine and dine And the Bentley came with the wings like a number nine, yeah Come through, just us two I like you ’cause you cut how I’m cut too Come through, just us two I like you ’cause you cut how I’m cut too [Chorus: Pop Smoke & Roddy Ricch] She wanna fuck with the Woo (Ayy, she wanna fuck with the Woo, Woo) She wanna fuck with the Woo She wanna fuck with the Woo She wanna fuck with the Woo Yeah, yeah, yeah, yeah [Verse 2: 50 Cent] That’s my kind of bitch, she be sayin’ some shit Like, “When you gon’ fly me in private, so I can land on that -

Lessons and Resources to Prepare for Worlds of Opportunity
Lessons and Resources to Prepare for Worlds of Opportunity 1 Table of Contents WOO Overview ................................................................................................................................... 1 Worlds of Opportunity Regions ........................................................................................................ 2 Lesson 1 Introduction to Alabama Worlds of Opportunity (AWOO)................................................ 5 Chalk Talk ........................................................................................................................................ 6 Employability Skill Positive Attitude.................................................................................................... 6 Lesson 2 Job Research ..................................................................................................................... 9 My Top Three Job Choices ............................................................................................................ 10 Employability Skill Teamwork ......................................................................................................... 10 Lesson 3 Job Interview Skills ......................................................................................................... 11 Interview Activities (Two days) ....................................................................................................... 12 Interview Questions ...…………………………….………………………………………………….………13 Ten Common Questions and Answers ………...….……………………...……………………………….14 -

4 Album Awards
4 ALBUM AWARDS Doja Cat Hot Pink RCA Victor Victor/ Meet The Woo 2 Pop Smoke Republic Records Rod Wave Ghetto Gospel Alamo Records Tones And I The Kids Are Coming Bad Batch/Elektra Records 12 MULTI-PLATINUM SINGLE AWARDS Arizona Zervas ROXANNE Columbia Jack Harlow WHATS POPPIN Atlantic Records Tones And I Dance Monkey Bad Batch/Elektra Records Doja Cat Say So RCA Gabby Barrett I Hope Warner Music Nashville 24kGoldn Mood (feat. iann dior) Records/Columbia Doja Cat Juicy RCA Victor Victor/ Dior Pop Smoke Republic Records death bed Powfu (coffee for your head) Columbia (feat. beabadoobee) Rod Wave Heart On Ice Alamo Records StaySolidRocky Party Girl Columbia Surfaces Sunday Best TenThousand Projects 20 PLATINUM SINGLE AWARDS 24kGoldn VALENTINO Records LLC/Columbia Ant Saunders Yellow Hearts Arista Baby Keem ORANGE SODA Baby Keem LLC Supalonely (feat. Republic Records BENEE Gus Dapperton) Conan Gray Heather Republic Records Conan Gray Maniac Republic Records Like That RCA Doja Cat (feat. Gucci Mane) Don Toliver No Idea Cactus Jack/Atlantic PTSD (feat. Juice Machine Entertainment G Herbo WRLD, Chance the Rapper & Lil Uzi Vert) Group LLC More Hearts Warner Music Nashville Ingrid Andress Than Mine JayDaYoungan 23 Island Ruffwayy John K if we never met Epic If The World Was Ending Arista JP Saxe (feat. Julia Michaels) Money Man 24 (feat. Lil Baby) Black Circle/EMPIRE For The Night Victor Victor/ Pop Smoke (feat. Lil Baby & DaBaby) Republic Records Mood Swings Victor Victor/ Pop Smoke (feat. Lil Tjay) Republic Records Ministry of Sound Regard Ride It Recordings/Epic ily (i love you baby) Astralwerks Surf Mesa (feat. -

Artist Song Album Blue Collar Down to the Line Four Wheel Drive
Artist Song Album (BTO) Bachman-Turner Overdrive Blue Collar Best Of BTO (BTO) Bachman-Turner Overdrive Down To The Line Best Of BTO (BTO) Bachman-Turner Overdrive Four Wheel Drive Best Of BTO (BTO) Bachman-Turner Overdrive Free Wheelin' Best Of BTO (BTO) Bachman-Turner Overdrive Gimme Your Money Please Best Of BTO (BTO) Bachman-Turner Overdrive Hey You Best Of BTO (BTO) Bachman-Turner Overdrive Let It Ride Best Of BTO (BTO) Bachman-Turner Overdrive Lookin' Out For #1 Best Of BTO (BTO) Bachman-Turner Overdrive Roll On Down The Highway Best Of BTO (BTO) Bachman-Turner Overdrive Take It Like A Man Best Of BTO (BTO) Bachman-Turner Overdrive Takin' Care Of Business Best Of BTO (BTO) Bachman-Turner Overdrive You Ain't Seen Nothing Yet Best Of BTO (BTO) Bachman-Turner Overdrive Takin' Care Of Business Hits of 1974 (BTO) Bachman-Turner Overdrive You Ain't Seen Nothin' Yet Hits of 1974 (ELO) Electric Light Orchestra Can't Get It Out Of My Head Greatest Hits of ELO (ELO) Electric Light Orchestra Evil Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Livin' Thing Greatest Hits of ELO (ELO) Electric Light Orchestra Ma-Ma-Ma Belle Greatest Hits of ELO (ELO) Electric Light Orchestra Mr. Blue Sky Greatest Hits of ELO (ELO) Electric Light Orchestra Rockaria Greatest Hits of ELO (ELO) Electric Light Orchestra Showdown Greatest Hits of ELO (ELO) Electric Light Orchestra Strange Magic Greatest Hits of ELO (ELO) Electric Light Orchestra Sweet Talkin' Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Telephone Line Greatest Hits of ELO (ELO) Electric Light Orchestra Turn To Stone Greatest Hits of ELO (ELO) Electric Light Orchestra Can't Get It Out Of My Head Greatest Hits of ELO (ELO) Electric Light Orchestra Evil Woman Greatest Hits of ELO (ELO) Electric Light Orchestra Livin' Thing Greatest Hits of ELO (ELO) Electric Light Orchestra Ma-Ma-Ma Belle Greatest Hits of ELO (ELO) Electric Light Orchestra Mr. -
THE WOO the HOME BUILDER an INDEPENDENT NEWSPAPER PUBLISHED in the INTEREST of WOODBRIDGE TOWNSHIP SEVENTEENTH YEAR Woodbridge, N
WOOBBMDO-E TOWNSHIP'S WOODBRIDGE TOWNSHIP'S, ' • FIVE. RAILROADS;" WELCOMES INVITE INDUSTRY THE WOO THE HOME BUILDER AN INDEPENDENT NEWSPAPER PUBLISHED IN THE INTEREST OF WOODBRIDGE TOWNSHIP SEVENTEENTH YEAR Woodbridge, N. J., Friday Afternoon, September 16, 1927 PRICE THREE CENTS—$1.50 PER YEAR DEMOCRATS JOHN TOOLAN DENOUNCE Shot Gun Welcome to New Theatre WLL DELIVER will be given patrons by Louise Glaum, famous "Vampire" A ctress of the Films, (in person), at premier performance. AT With the top of his head NEW THEATRE 2, 3, 4 blown off from the discharge .^•F o qhutfim, the muzzle of Lavin, ct Making, Es which he placed in his mouth, Senator Larson Will be Mas- How Republican Ole Clausen. 34, a painter, liv- ter of Ceremonies. Cinema ing at 649 Hazel street, Perth "Vampire" Will;Speak: on Seeks to Use Constitution Amboy, was found this fore- as Cat's Paw. noon at the corner of King Hollywood Studios. Genrp-e's road and West Pond - UBP'S TUP • If ATI? road by two young men, Jack Jacobson and George Larson, Party Leaders of Comity Fear.bot£ °f Hopeiawn. - _ "lieaire Beautiful" Will ». .1 c IT , Tsr-n rs i No letters or notes giving a Give Public Best Films and Apathy Of Voters Will Re-!motive for the suicide were salt In Coup by Power- found on his person. Clausen Splendid Music, Says Man- was married and leaves a wife] ager Marcus. • ',"• •••'':• and two small children. The The Woodbridse Township Demo- ib °dy wawass ggive n in custodyy of I One of the most populai- officials cratic Club, at a meeting in Hun-1 Undertaker Flynn, • of Wood- of the county, Prosecutor John E. -

From Pieces to Weight: Once Upon a Time in Southside Queens Free
FREE FROM PIECES TO WEIGHT: ONCE UPON A TIME IN SOUTHSIDE QUEENS PDF 50 Cent,Kris Ex | 223 pages | 15 Aug 2006 | SIMON & SCHUSTER | 9780743488044 | English | New York, NY, United States Verify your identity His mother, a smalltime drug dealer, was murdered when he was eight, but that didn't stop him from pursuing her profession. In his neighborhood, recalls 50 Cent, the only people with money were the drug dealers: "They were my role models. Over the next decade, 50 Cent evolved from a hustler selling capsules of crack cocaine "pieces" to a kingpin purchasing by the kilo "weight". With money came girls, clothes, cars—and trouble. He avoided jail by serving time in a boot camp—style incarceration center, which did nothing but turn him into a "stronger, meaner, and more focused criminal. Yet From Pieces to Weight: Once Upon a Time in Southside Queens while trying to break into the business, 50 Cent couldn't leave his past behind, wearing bulletproof vests to meetings with record executives and slipping back into the drug trade when his career didn't take off immediately. Fans will discover the origins of 50 Cent's famous feud with Ja Rule; they'll also get more details about the notorious shooting that left him with nine From Pieces to Weight: Once Upon a Time in Southside Queens holes in his body. Opinionated, unrepentant and unabashedly self-promoting, 50 Cent's memoir celebrates the rapper's peculiar brand of the American Dream and the title of his breakthrough album : Get Rich or Die Tryin'. -

Joseph Clarence Woo Jr. MD, FACP, FACC, FCCP and His Wife Mabel Have Been
Joseph Clarence Woo Jr. MD, FACP, FACC, FCCP and his wife Mabel have been the gracious donors of several major gifts and untold hours of dedicated time and commitment to the overall development of Community Medical Centers’ hospitals and facilities. Life is circuitous. The circle of life and death, the circles of friendship and mutual commitment, relationships that span decades and generations. Even the circuitous path blood travels as it passes through the right and left ventricles of the heart. Life-giving, life-affirming circles. This is the story of a lifetime of relationships, a vast, connected series of circles. 1900 was a simpler time. In California, it was a time of gold rush bust, railroad boom and agricultural prosperity. In Fresno, it was a time of street cars and Piggly Wigglys. It was a time when Fresno’s largest health care provider was a boarding house-turned-hospital. Celia Burnett, proprietor of that successful Fresno boarding house, had been approached by several physicians about turning her boarding house into a private hospital. She agreed and the Burnett Sanitarium, at the southeast corner of Fulton and Calaveras streets, served the community of Fresno from 1897 to 1905. The Fresno Morning Republican newspaper reported that the mission of the hospital was “to provide a place where persons suffering from disease can be treated while under the care of physicians.” It was a simple objective for a simple time. Specialty care was limited, doctors still made house calls, and most babies were born at home. The use of X-rays was still being explored. -
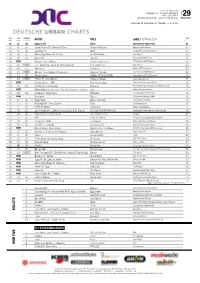
Bullets High Five
DUC REDAKTION Hofweg 61a · D-22085 Hamburg T 040 - 369 059 0 WEEK 29 [email protected] · www.trendcharts.de 09.07.2020 Approved for publication on Tuesday, 14.07.2020 THIS LAST WEEKS IN PEAK WEEK WEEK CHARTS ARTIST TITLE LABEL/DISTRIBUTOR POSITION 01 01 08 Apache 207 Fame TwoSides/Four Music/Sony 01 02 07 05 Scott Storch Ft. Ozuna & Tyga Fuego Del Calor Atlantic/WMI/Warner 02 03 03 04 Apache 207 Boot TwoSides/Four Music/Sony 03 04 04 03 Black Eyed Peas Ft. El Alfa NO MAÑANA Epic/Sony 04 05 08 06 Dojo Cat Say So Kemosabe/RCA/Sony 05 06 NEW Megan Thee Stallion Girls In The Hood 300/Atlantic/WMI/Warner 06 07 02 10 THE SCOTTS, Travis Scott & Kid Cudi THE SCOTTS Epic/Sony 02 08 09 07 Pashanim Airwaves Urban/VEC/Universal 08 09 21 10 Megan Thee Stallion & Beyoncé Savage (Remix) 300/Atlantic/WMI/Warner 08 10 14 05 Shirin David HOES UP G'S DOWN Juicy Money/VEC/Universal 10 11 23 10 T-Pain Ft. Chris Brown Wake Up Dead Nappy Boy/Empire 11 12 NEW Kynda Grey Ft. RIN Ayo Technology Division/Gold League/Sony 12 13 10 04 Lil Wayne Ft. Doja Cat Shimmy Young Money/Cash Money/UMI/Universal 10 14 NEW Miksu/Maclou, Summer Cem & Luciano Ft. Jamule XXL Futura/Groove Attack 14 15 26 03 6ix9ine Ft. Nicki Minaj TROLLZ Scumgang/Create Music 15 16 NEW Saweetie Tap In ICY/Artistry/Warner/WMI 16 17 34 05 > Sean Paul Back It Up Deh Dutty Rock/ONErpm 10 18 11 05 ROSALÍA Ft.