Introduction to Mauve - Updated: 11 November 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMPLETE WARDROBE of SHADES. for BEST RESULTS, Dr.’S REMEDY SHADE COLLECTION SHOULD BE USED TOGETHER with BASIC BASE COAT and CALMING CLEAR SEALING TOP COAT
COMPLETE WARDROBE OF SHADES. FOR BEST RESULTS, Dr.’s REMEDY SHADE COLLECTION SHOULD BE USED TOGETHER WITH BASIC BASE COAT AND CALMING CLEAR SEALING TOP COAT. ALTRUISTIC AMITY BALANCE NEW BOUNTIFUL BRAVE CHEERFUL CLARITY COZY Auburn Amethyst Brick Red BELOVED Blue Berry Cherry Coral Cafe A playful burnt A moderately A deep Blush A tranquil, Bright, fresh and A bold, juicy and Bright pinky A cafe au lait orange with bright, smokey modern Cool cotton candy cornflower blue undeniably feminine; upbeat shimmer- orangey and with hints of earthy, autumn purple. maroon. crème with a flecked with a the perfect blend of flecked candy red. matte. pinkish grey undertones. high-gloss finish. hint of shimmer. romance and fun. and a splash of lilac. DEFENSE FOCUS GLEE HOPEFUL KINETIC LOVEABLE LOYAL MELLOW MINDFUL Deep Red Fuchsia Gold Hot Pink Khaki Lavender Linen Mauve Mulberry A rich A hot pink Rich, The perfect Versatile warm A lilac An ultimate A delicate This renewed bordeaux with classic with shimmery and ultra bright taupe—enhanced that lends everyday shade of juicy berry shade a luxurious rich, romantic luxurious. pink, almost with cool tinges of sophistication sheer nude. eggplant, with is stylishly tart matte finish. allure. neon and green and gray. to springs a subtle pink yet playful sweet perfectly matte. flirty frocks. undertone. & classic. MOTIVATING NOBLE NURTURE PASSION PEACEFUL PLAYFUL PLEASING POISED POSITIVE Mink Navy Nude Pink Purple Pink Coral Pink Peach Pink Champagne Pastel Pink A muted mink, A sea-at-dusk Barely there A subtle, A poppy, A cheerful A pale, peachy- A high-shine, Baby girl pink spiked with subtle shade that beautiful with sparkly fresh bubble- candy pink with coral creme shimmering soft with swirls of purple and cocoa reflects light a hint of boysenberry. -

Color Chart Colorchart
Color Chart AMERICANA ACRYLICS Snow (Titanium) White White Wash Cool White Warm White Light Buttermilk Buttermilk Oyster Beige Antique White Desert Sand Bleached Sand Eggshell Pink Chiffon Baby Blush Cotton Candy Electric Pink Poodleskirt Pink Baby Pink Petal Pink Bubblegum Pink Carousel Pink Royal Fuchsia Wild Berry Peony Pink Boysenberry Pink Dragon Fruit Joyful Pink Razzle Berry Berry Cobbler French Mauve Vintage Pink Terra Coral Blush Pink Coral Scarlet Watermelon Slice Cadmium Red Red Alert Cinnamon Drop True Red Calico Red Cherry Red Tuscan Red Berry Red Santa Red Brilliant Red Primary Red Country Red Tomato Red Naphthol Red Oxblood Burgundy Wine Heritage Brick Alizarin Crimson Deep Burgundy Napa Red Rookwood Red Antique Maroon Mulberry Cranberry Wine Natural Buff Sugared Peach White Peach Warm Beige Coral Cloud Cactus Flower Melon Coral Blush Bright Salmon Peaches 'n Cream Coral Shell Tangerine Bright Orange Jack-O'-Lantern Orange Spiced Pumpkin Tangelo Orange Orange Flame Canyon Orange Warm Sunset Cadmium Orange Dried Clay Persimmon Burnt Orange Georgia Clay Banana Cream Sand Pineapple Sunny Day Lemon Yellow Summer Squash Bright Yellow Cadmium Yellow Yellow Light Golden Yellow Primary Yellow Saffron Yellow Moon Yellow Marigold Golden Straw Yellow Ochre Camel True Ochre Antique Gold Antique Gold Deep Citron Green Margarita Chartreuse Yellow Olive Green Yellow Green Matcha Green Wasabi Green Celery Shoot Antique Green Light Sage Light Lime Pistachio Mint Irish Moss Sweet Mint Sage Mint Mint Julep Green Jadeite Glass Green Tree Jade -

Colour of Emotions
Colour of Emotions Utilizing The Psychology of Color GRA 3508 - Desktop Publishing II Table of Contents Introduction ........................................................................... 2 Psychology of Logos ............................................................... 3 Reds ....................................................................................... 4 Overview of Red,Mauve, Magenta ............................................... 4 Crimson, Scarlet, Poster Red ...................................................... 5 Yellows................................................................................... 6 Overview of Yellow, Coral, Orange ............................................... 6 Amber, Gold, Yellow ................................................................. 7 Greens ................................................................................... 8 Overview of Green, Lime, Leaf Green .......................................... 8 Sea Green, Emerald, Teal .......................................................... 9 Blues ..................................................................................... 10 Overview of Blue, Cyan, Sky Blue ............................................... 10 Ultramarine, Violet, Purple ......................................................... 11 Credits.................................................................................... 12 1 Introduction Psychology of Logos Color is a magical element that gives feeling and emotion to art and design. It is an exclusive -

COLOR SELECTION High Gloss Colors Matte Finish Colors Choosing a Color Is a Very Personal Decision, So QUICKIE and ZIPPIE Give You More Options
POWER CHAIR SHROUD COLORS & COLOR SELECTION High Gloss Colors Matte Finish Colors Choosing a color is a very personal decision, so QUICKIE and ZIPPIE give you more options. From subtle to eye-catching, we offer the Apple Green Copper Silver Matte Black widest spectrum of possibilities. Explore our collection to find the look that suits you! Special Edition White Candy Apple Red High Gloss Black Yellow Digital Camo Available exclusively on QUICKIE 7 R, 7RS and Q7 NextGEN KOLORFUSION ™ Candy Blue Midnight Blue American Flag Woodland Camo Desert Camo Mossy Oak Camouflage Stars and Stripes Candy Purple Pearl Pink Pink Camo Grunge Skulls Zebra* Color match your * Black Zebra Kolorfusion ™ pattern is available on any base color. JAY ® J3 Back to your Shown here on Candy Blue base color. Quickie ® chair! Carbon Fiber Pearl White ©01.2016 Sunrise Medical (US) LLC Available for order MK-100168 Rev. B through JAY Your Way ™ customizations Customer Service: 800.333.4000 www.SunriseMedical.com FRAME COLORS Sparkle Silver, Red Anodized Package Zebra Kolorfusion ™ Glow paint shown in the dark High Gloss Colors Matte Finish Colors Aztec Gold Candy Red Rootbeer Titanium Color Paint Black Electric Blue Sparkle Silver Matte Black Black Cherry Evergreen Sunrise Orange Matte Black Cherry Black Opal Glow Yellow Matte Purple ANODIZED PARTS Blue Opal Green Apple Refer to product order form for anodized part options. Matte Electric Blue Candy Blue Hot Sparkle Pink Black Titanium Grey Gold Blue Matte Evergreen NOTES: Print processes cannot accurately represent paint finishes. Actual colors may vary. Candy Purple Mauve Pink Colors and patterns may vary based on type of chair or frame material. -

Fax) [email protected]
2018 Our 24th year Macey and Greg McCullough 800-934-4747 800-934-4747 (Fax) [email protected] www.iriscitygardens.com Contents General information Call or visit................................................................ 3 Directions to the gardens........................................... 3 Ordering and payment............................................... 4 Gift certificates.......................................................... 4 Shipping.................................................................... 4 Substitutes................................................................. 5 Guarantee.................................................................. 5 Iris awards and terminology .................................................... 6 New Introductions ................................................................... 7 Garden Markers....................................................................... 9 Louisiana iris ......................................................................... 10 Other beardless iris: I. anguifuga ............................................................. 26 I. laevigatae ............................................................. 26 I. pseudacorus ......................................................... 27 I. tectorum ............................................................... 27 I. versicolor ............................................................. 27 Species Crosses....................................................... 28 Bearded iris........................................................................... -

SPECIES IDENTIFICATION GUIDE National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE
National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE Contents White / Cream ................................ 2 Grasses ...................................... 130 Yellow ..........................................33 Rushes ....................................... 138 Red .............................................63 Sedges ....................................... 140 Pink ............................................66 Shrubs / Trees .............................. 148 Blue / Purple .................................83 Wood-rushes ................................ 154 Green / Brown ............................. 106 Indexes Aquatics ..................................... 118 Common name ............................. 155 Clubmosses ................................. 124 Scientific name ............................. 160 Ferns / Horsetails .......................... 125 Appendix .................................... 165 Key Traffic light system WF symbol R A G Species with the symbol G are For those recording at the generally easier to identify; Wildflower Level only. species with the symbol A may be harder to identify and additional information is provided, particularly on illustrations, to support you. Those with the symbol R may be confused with other species. In this instance distinguishing features are provided. Introduction This guide has been produced to help you identify the plants we would like you to record for the National Plant Monitoring Scheme. There is an index at -

COLORS Glitter Sneakers Signature Silk Slip Dress White Button Lipstick Down BRAND GEL POLISH
PARTY READY • HOLIDAY 2021 169 COLORS Glitter Sneakers Signature Silk Slip Dress White Button Lipstick Down BRAND GEL POLISH Cream Puff White Wedding White Button Lady Lilly Studio White Bouquet Naked Naiveté Satin Slippers Mover & Shaker Ice Bar Negligee Down Romantique Pointe Blanc Beau Aurora Winter Glow Unlocked Clearly Pink Uncovered Unmasked Grapefruit Soft Peony Bare Chemise Baby Smile Sparkle Rule Breaker Pink Pursuit Salmon Run Jellied Glitter Sneakers Exquisite Bellini Powder My Nose Wrapped in Sweet Cider Satin Pajamas Flowerbed Folly Chandelier Linen Boheme Iced Cappuccino Clay Canyon Self-Lover Silk Slip Dress Gala Girl Cashmere Wrap Field Fox Nude Knickers Radiant Chill Tundra Fragrant Freesia Candied Be Demure Blushing Topaz Blush Teddy Strawberry Lavender Lace Beckoning Mauve Maverick Coquette Cake Pop Pacifi c Rose Rose Bud Kiss From A Gotcha Smoothie Begonia Rose Holographic Married To The Wooded Bliss Fuji Love Untitled Bronze Sultry Sunset Rooftop Hop Magenta Mischief Tutti Frutti Hot Pop Pink Ecstasy Pink Bikini Museum Meet Mauve Cute Pink Leggings Offbeat Lobster Roll Jelly Bracelet Charm Tropix Beach Escape Sparks Fly Desert Poppy Uninhibited Catch Of The B-Day Candle Mambo Beat Day Soulmate Hollywood Liberté Sangria At Femme Fatale Kiss The Skipper Element Kiss Of Fire Wildfi re Hot Or Knot Soft Flame Devil Red First Love Sunset Hot Chilis Bordeaux Babe Books & Brick Knit Company Red Tartan Punk Rose Brocade Red Baroness Ripe Guava Ruby Ritz Garnet Glamour How Merlot Rouge Rite Beaujolais Rebellious Ruby Decadence -

Qualatex Rainbow and Custom Colors
Rainbow of Colors Diamond White Pearl Gray Silver Pearl Ivory Pearl Yellow Citrine Pearl Clear White Ivory Silk Lemon Chion Yellow Citrine Yellow Goldenrod Gold Blush Neon Mocha Chocolate Pearl Rose Coral Orange Mandarin Orange Brown Brown Peach Gold Orange Pearl Mandarin Pearl Pink Neon Neon Rose Wild Pearl Jewel Red Ruby Red Orange Pink Pink Magenta Berry Magenta Magenta Pearl Maroon Sparkling Pearl Pearl Neon Spring Purple Quartz Pearl Pearl Ruby Red Burgundy Burgundy Lavender Violet Lilac Violet Purple Quartz Purple Light Blue Pearl Pale Neon Robin’s Periwinkle Dark Sapphire Pearl Pearl Navy Caribbean Azure Blue Blue Egg Blue Blue Blue Sapphire Blue Midnight Blue Blue Tropical Jewel Pearl Pearl Neon Wintergreen Lime Jewel Pearl Lime Spring Green Teal Teal Teal Mint Green Green Green Lime Green Green C h r o m e B all o on s Emerald Pearl Pearl Pearl Onyx Black Chrome Chrome Chrome Chrome Chrome Chrome Green Emerald Green Forest Green Onyx Black Silver Gold Mauve Purple Blue Green S u p e r A g a t e Traditional Fashion Red & Black & Yellow Orange Pink Violet Red Orange Blue Green White White Rainbow Rainbow Rainbow Rainbow Rainbow LEGEND Custom Colors Outside Balloon ® Inside Balloon Use the extensive Qualatex Rainbow of Colors as a palette from which to create custom colors by “layering” p two different balloons. This “double-stuffing” technique yields an unlimited number of unique colors and finishes. Custom Balloon Pearl White White Pearl Citrine Yellow Pearl White Gold Pearl Ivory Pearl Peach Ivory Silk Pearl Peach Mandarin Orange -

SNS Products Color Guide
SNS Products VOLUME 1 Color Guide 07.28.19 TABLE OF CONTENTS Welcome to Our SNS Gelous Colors ...........................................................4 Birds of Paradise Collection.................................................7 World of Color Blooming Meadow Collection ..............................................7 Winter Wonderland Collection ..............................................8 At SNS, we love color—that’s why Phone Indian Summer Collection ...................................................8 (888) 445-2786 we provide a wider choice, with C’est La Vie Collection .......................................................9 Social Media Autumn Collection ............................................................9 Facebook.com/snsnailsproduct more healthy product options, than Instagram.com/snsnailsproduct/ Nude in Spring Collection ................................................. 10 any other company. Pinterest.com/snsnailsproduct Best of Spring Collection .................................................. 10 Website Bridal Collection............................................................. 11 Explore the choices in this special snsnails.com Cleopatra Collection........................................................ 11 Email Fairytale Collection ......................................................... 11 SNS Color Guide, then get your PRODUCT ORDERS: Summer Collection ......................................................... 11 hands on the latest and greatest (Authorized Distributors Only) Diva Collection ............................................................. -

Introduction to Mauve - Updated: 21 July 2008
Introduction to Mauve - Updated: 21 July 2008 Introduction to Mauve Genomes evolve Over the course of evolution, genomes can undergo many small and large-scale changes. Local changes such as nucleotide substitution and indels have been observed in comparative studies of individual genes. Recombination, however, can cause large-scale changes such as gene loss, duplication, rearrangement, and horizontal transfer. Understanding the rates and patterns of each type of change is expected to yield insights into various biological processes. Genome Alignment - A means for comparison The advent of genome sequencing provides the data needed to characterize rates and patterns of genome evolution. Genome alignments can identify evolutionary changes in the DNA by aligning homologous regions of sequence. Mauve is a software package that attempts to align orthologous and xenologous regions among two or more genome sequences that have undergone both local and large-scale changes. Locally Collinear Blocks Because recombination can cause genome rearrangements, orthologous regions of one genome may be reordered or inverted relative to another genome. During the alignment process, Mauve identifies conserved segments that appear to be internally free from genome rearrangements. Such regions are referred to as Locally Collinear Blocks (LCBs). The original Mauve algorithm This section gives a brief working overview of the Mauve algorithm. For more detail, please see the paper Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements that appears in Genome Research . Anchored alignment Like many other genome alignment methods, Mauve uses the anchored alignment technique to rapidly align genomes. Unlike most genome alignment methods however, Mauve allows the order of alignment anchors to be rearranged in each genome permitting identification of genome rearrangements. -
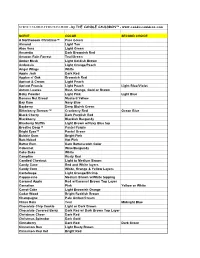
SCENT / COLOR SUGGESTION CHART - by the CANDLE CAULDRON™
SCENT / COLOR SUGGESTION CHART - by THE CANDLE CAULDRON™ - www.candlecauldron.com SCENT COLOR SECOND CHOICE A Northwoods Christmas™ Pine Green Almond Light Tan Aloe Vera Light Green Amaretto Dark Brownish Red Amazon Rain Forrest Teal/Green Amber Musk Light Goldish Brown Ambrosia Light Orange/Peach Angel Wings White Apple Jack Dark Red Apples n' Oak Brownish Red Apricot & Cream Light Peach Apricot Freesia Light Peach Light Blue/Violet Autum Leaves Rust, Orange, Gold or Brown Baby Powder Light Pink Light Blue Banana Nut Bread Mustard Yellow Bay Rum Navy Blue Bayberry Deep Bluish Green Bitterberry Breezer™ Cranberry Red Ocean Blue Black Cherry Dark Purplish Red Blackberry Blackish Burgundy Blueberry Muffin Light Brown w/Navy Blue top Breathe Deep™ Pastel Purple Bright Eyes™ Pastel Green Bubble Gum Bright Pink Butt Naked Hot Pink Butter Rum Dark Butterscotch Color Cabernet Wine/Burgundy Cake Bake White Campfire Rusty Red Candied Chestnut Light to Medium Brown Candy Cane Red and White layers Candy Corn White, Orange & Yellow Layers Canteloupe Light Orange/Shrimp Cappuccino Medium Brown w/White topping Caramel Apple Red w/Caramel Brown Top Layer Carnation Pink Yellow or White Carrot Cake Light Brownish Orange Cedar Wood Bright Reddish Brown Champagne Pale Amber/Cream China Rain Teal Midnight Blue Chocolate Chip Cookie Light or Dark Brown Chocolate Covered Berry Dark Red w/ Dark Brown Top Layer Christmas Cheer Dark Red Christmas Splendor Dark Gold Cinnaberry Dark Red Dark Green Cinnamon Bun Light Rusty Brown Cinnamon Red Hot Bright Red Cinnamon -

Floriani Thread Color Chart
Floriani Deluxe Thread Color Chart High Sheen 100% Polyester Thread Available in 1000M and 5000M Cones 1 Copper Black Cherry Begonia Light Pink Sangria Deep Violet PF0186 PMS 179C PF0197 PMS 195C PF1083 PMS 191C PF0102 PMS 1895C PF0139 PMS 229C PF0665 PMS 273C 2 Wildflower Old Roseleaf Shrimp Oyster Powder Puff Grape Juice PF0173 PMS 172C PF0196 PMS 506C PF1107 PMS 148C PF0100 PMS Cool Gray 1C PF0133 PMS 514C PF0696 PMS 2685 3 Burnt Orange Cabernet Grapefruit Pale Peach Roseleaf Grape Jam PF0755 PMS 166C PF1586 PMS 1815C PF0155 PMS 1925C PF0110 PMS 503C PF1902 PMS 687C PF0695 PMS 2685C 4 Orange Russet Rose Cerise Rosewater Pink Mist Tulip PF0172 PMS 165C PF0195 PMS 194C PF1082 PMS 190C PF0161 PMS 705C PF0123 PMS 2365C PF0694 PMS 2617C 5 Carrot Cherry Blush Soapstone Wisteria Amethyst PF0537 PMS 151C PF0193 PMS 194C PF0153 PMS 701C PF0163 PMS 488C PF0130 PMS 678C PF0672 PMS 2567C 6 Golden Poppy Scarlet Misty Pink Buff Bright Pink Lavender PF0535 PMS 164C PF0190 PMS 201C PF0117 PMS 701C PF1021 PMS 698C PF0125 PMS 223C PF0673 PMS 2577C 7 Bijol Burgundy Candy Pink Lace Light Lilac Light Mauve PF0526 PMS 1375C PF0194 PMS 202C PF0152 PMS 700C PF0116 PMS 496C PF0131 PMS 236C PF0674 PMS 2587C 8 Sorbet Deep Rust Champagne Light Coral Hot Pink Luxury PF0525 PMS 137C PF0192 PMS 207C PF1011 PMS 706C PF0140 PMS 169C PF0127 PMS 191C PF0675 PMS 526C 9 Apricot Hibiscus Dusty Rose Pink Rose Flamingo Royal Purple PF0533 PMS 137C PF1084 PMS 1925C PF1014 PMS 710C PF1081 PMS 707C PF0128 PMS 240C PF0676 PMS 2617C 10 Pumpkin Persimmon China Rose Pale Pink Deep